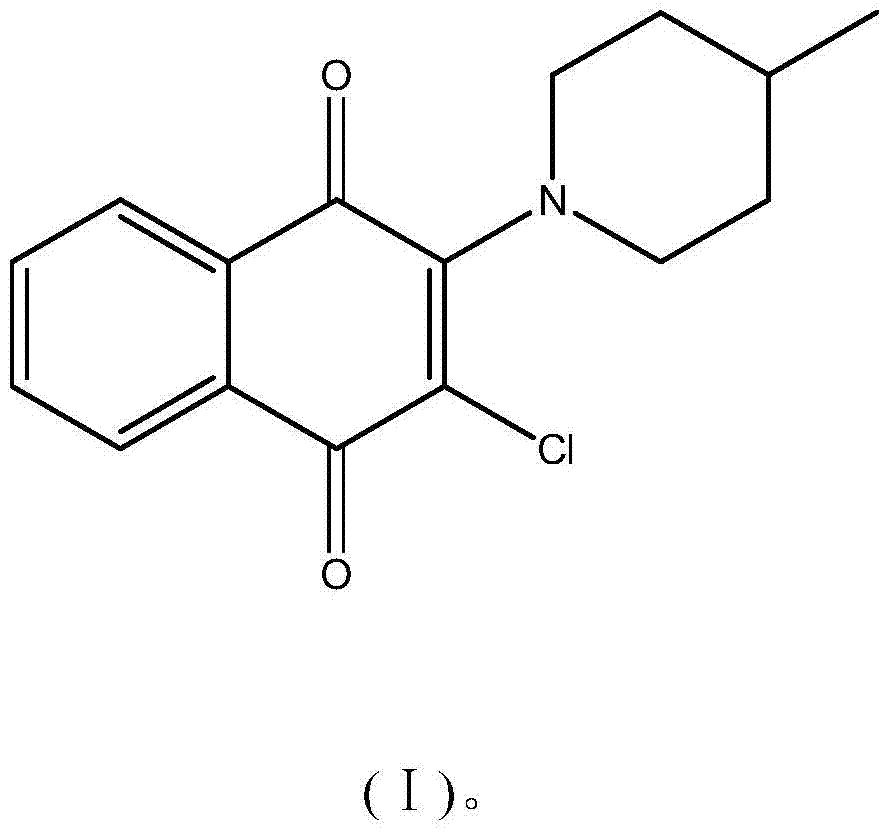
Application of a naphthalene-1,4-diketone compound as an hcbs enzyme inhibitor
A technology of compounds and diketones, applied in the application field of hCBS enzyme inhibitors, can solve the problems of unclear amino acid residues and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Embodiment 1, source truncated hCBS △ Expression and purification of 414-551 (hCBS-413) expression and purification method
[0065] According to Frank, N., in Arch Biochem Biophys470,64-72 (2008), Oliveriusova, J., J Biol Chem277, 48386-94 (2002). Or Janosik, M. et al. in Acta Crystallogr D BiolCrystallogr57, 289- 91 (2001), the method described in, first hCBS △ 414-551hCBS-413) gene was cloned into glutathione S-transferase (GST) fusion expression vector pGEX-KG, then overexpressed and then affinity-purified using GST-agarose column.
[0066] Sequence Listing SEQ ID No: 1 is the amino acid sequence of hCBS enzyme;
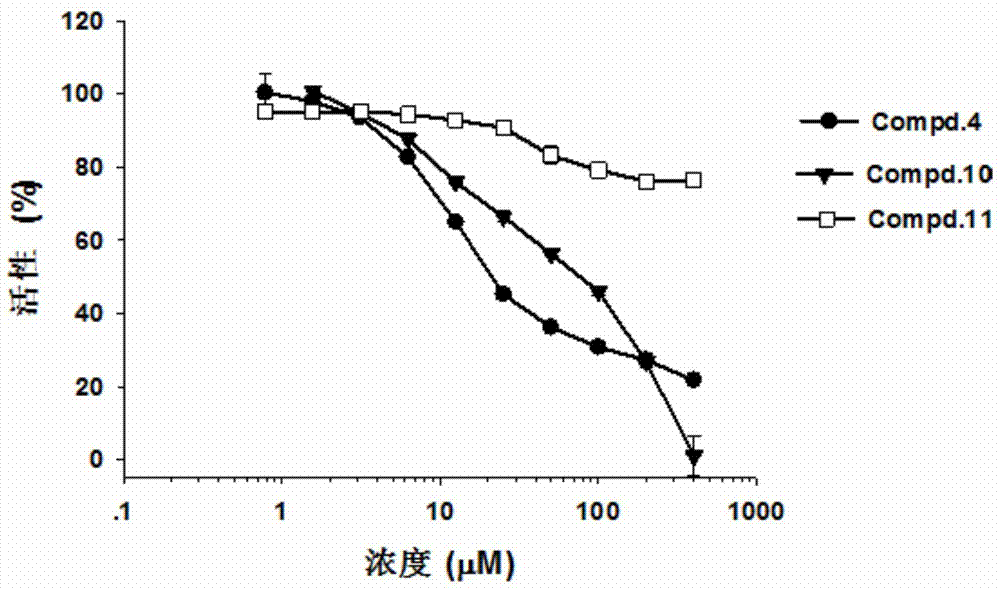
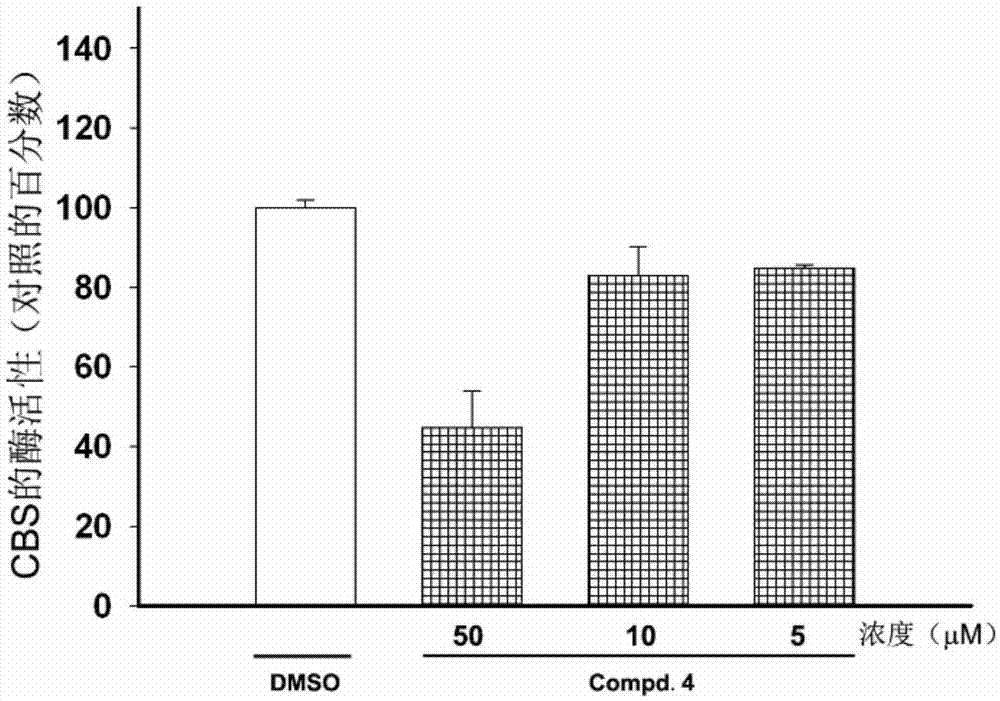
[0067] Use of compound Compd.4 as hCBS enzyme inhibitor:
Embodiment 2
[0068] Example 2. Determination of the inhibition rate of different concentrations of compound Compd.4 on hCBS enzyme activity:
[0069] Step 1. Preparation of buffer solution: add Tris-HCl concentration of 50mM, PLP concentration of 100μM, hCBS-413 concentration of 100nM, L-Cys concentration of 4mM, D, L-HCys concentration of 4mM to the enzymatic reaction vessel Ionic water solution, pH=8.6;
[0070] Step 2. Prepare the enzymatic reaction mixture: add compound Compd.4 to the buffer solution prepared in step 1, and prepare compound Compd.4 at concentrations of 400 μM, 200 μM, 100 μM, 50 μM, 25 μM, 12.5 μM, 6.2 μM, 3.125μM, 1.56μM, 0.78μM enzymatic reaction mixture to form an enzymatic reaction system;
[0071] Step three, preparation of H 2 S gas detection system:
[0072] in H 2 Add 50 μL of DTNB solution into the S gas detection container, and the DTNB solution is a deionized aqueous solution with a concentration of DTNB of 300 μM, a concentration of Tris-HCl of 262 mM, ...
Embodiment 3
[0079] Example 3. Determination of the inhibition rate of different concentrations of compound Compd.10 on hCBS enzyme activity;
[0080] Step 1. Preparation of buffer solution: add Tris-HCl concentration of 50mM, PLP concentration of 100μM, hCBS-413 concentration of 100nM, L-Cys concentration of 4mM, D, L-HCys concentration of 4mM to the enzymatic reaction vessel Ionic water solution, pH=8.6;
[0081] Step 2. Prepare the enzymatic reaction mixture: add compound Compd.10 to the buffer solution prepared in step 1, and prepare compound Compd.10 at concentrations of 400 μM, 200 μM, 100 μM, 50 μM, 25 μM, 12.5 μM, 6.2 μM, 3.125μM, 1.56μM, 0.78μM enzymatic reaction mixture to form an enzymatic reaction system;
[0082] Step three, preparation of H 2 S gas detection system:
[0083] in H 2 Add 50 μL of DTNB solution into the S gas detection container, and the DTNB solution is a deionized aqueous solution with a concentration of DTNB of 300 μM, a concentration of Tris-HCl of 262 m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com