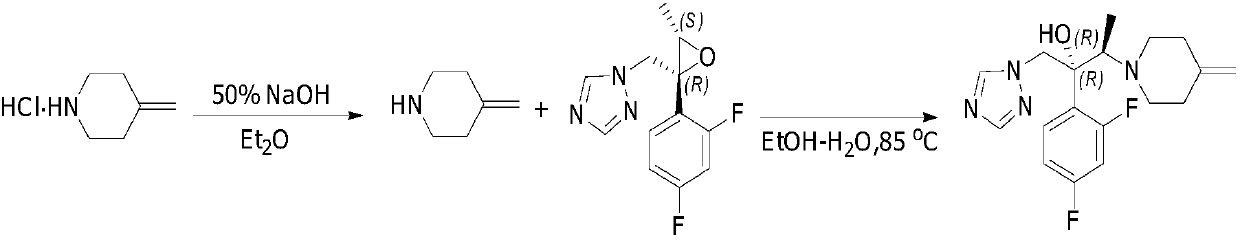
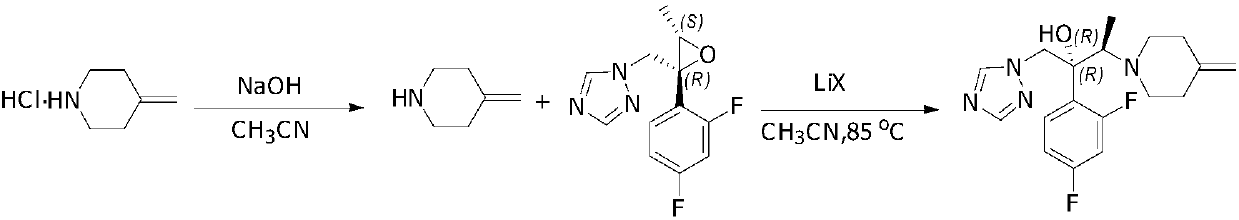
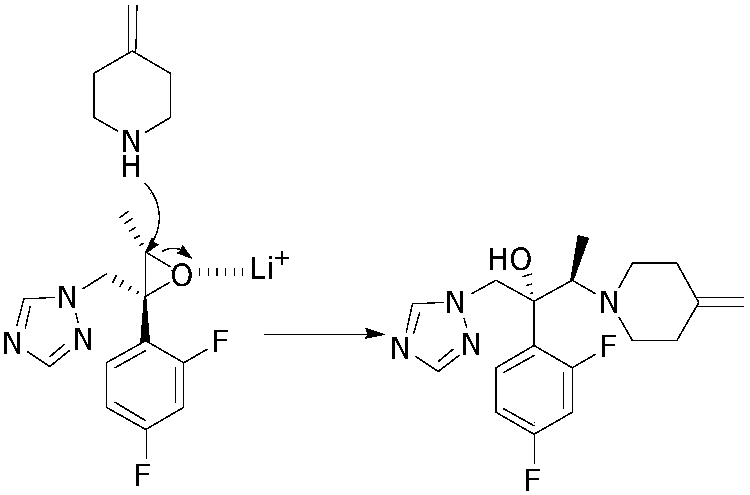
Preparing method of 1-triazole-2-butanol derivative
A technology of derivatives and butanol, which is applied in the field of preparation of 1-triazole-2-butanol derivative ffluconazole, can solve the problems of low product purity, low yield and many impurities, and achieves selectivity Good, high yield, complete reaction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] In a 250mL three-neck flask, equipped with a thermometer, add 4-methylene piperidine hydrochloride (25.7g, 199mmol), NaOH (8g, 199mmol), 80mL acetonitrile, and stir for 30 minutes at 25°C. Then add (2R, 3S) -2-(2,4-Difluorophenyl)-3-methyl-[(1H-1,2,4-triazol-1-yl)methyl]oxirane (20g, 79.6mmol) , LiCl (8.4g, 199mmol). 85-90°C for 22-25 hours. TLC shows that the reaction is complete. Post-treatment: stop heating, cool to room temperature, filter, wash the filter cake with 50 mL of acetonitrile, wash the organic phase with 50 mL of water, separate liquids, concentrate and dry to obtain 35 g of oil. Then recrystallize from ethanol / water. The white solid obtained by drying is efluconazole: 25.7 g, yield: 93.0%, purity: 99.5%.
[0030] Obtained solid 1 H NMR(300MHz, CDCl 3 ): δ8.00(s,1H),7.75(s,1H),7.51-7.45(m,1H),6.78-6.68(m,2H),5.44(s,1H), 4.82(dd,J=18.0 ,12.0Hz,2H),4.61(s,2H),2.90-2.86(m,1H),2.69-2.66(m,2H),2.32(br,2H),2.21-2.17(m,4H),0.92( dd,J=6.0,3.0Hz,3H); 13CNMR (75M...
Embodiment 2
[0032] In a 250mL three-neck flask, equipped with a thermometer, add 4-methylene piperidine hydrochloride (25.7g, 199mmol), NaOH (8g, 199mmol), 80mL acetonitrile, and stir for 30 minutes at 25°C. Then add (2R, 3S) -2-(2,4-Difluorophenyl)-3-methyl-[(1H-1,2,4-triazol-1-yl)methyl]oxirane (20g, 79.6mmol) , LiBr (17.3g, 199mmol). The reaction was conducted at 85-90°C for 5 hours. TLC indicated that the reaction was complete. Post-treatment: stop heating, cool to room temperature, filter, wash the filter cake with 50 mL of acetonitrile, wash the organic phase with 50 mL of water, separate, concentrate and dry, use ethanol 50 mL*2 twice to obtain 36 g of oil. Then recrystallize from ethanol / water. The white solid obtained by drying is efluconazole: 25.9 g, yield: 93.7%, purity: 99.6%.
Embodiment 3
[0034] In a 250mL three-neck flask, equipped with a thermometer, add 4-methylene piperidine hydrochloride (25.7g, 199mmol), NaOH (8g, 199mmol), 80mL acetonitrile, and stir for 30 minutes at 25°C. Then add (2R, 3S) -2-(2,4-Difluorophenyl)-3-methyl-[(1H-1,2,4-triazol-1-yl)methyl]oxirane (20g, 79.6mmol) , LiI (26g, 199mmol), reacted at 85-90°C for 5 hours. TLC indicated that the reaction was complete. Post-treatment: stop heating, cool to room temperature, filter, wash the filter cake with 50 mL of acetonitrile, wash the organic phase with 50 mL of water, separate and concentrate to dry to obtain 38 g of oil. Then recrystallize from ethanol / water. The white solid obtained by drying is efluconazole: 26.5 g, yield: 96%, purity: 99.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com