Preparation method for Xeljanz related substance
A technology of tofacitinib and related substances, which is applied in the field of medicinal chemistry, can solve the problems of high cost and limited industrial application, and achieve the effects of simple operation, mild reaction conditions and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
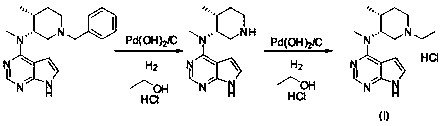
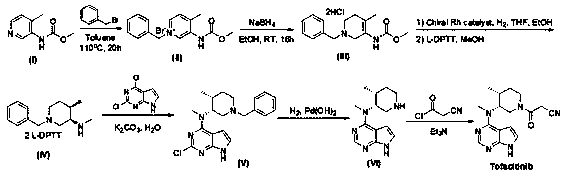
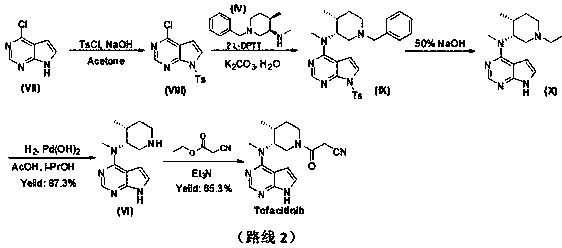
[0032] Add N-methyl-N-((3R,4R)-4-methylpiperidin-3-yl)-7H-pyrrolo[2,3-d]pyrimidine-4 to a 250ml three-necked flask at 25°C -Amine hydrochloride (6.0g, 21.3mmol), add methanol (60ml), stir to dissolve, then add formic acid (2.2g, 32.0mmol), acetaldehyde (10.8g, 213mmol), then add NaBH in three batches 4 (1.6g, 42.6mmol), TLC (DCM:MeOH=10:1) after 1h reaction, the reaction was complete. The reaction solution was concentrated to dryness, water (60ml) and EA (60ml) were added to the concentrate, the layers were stirred, the water layer was extracted once with EA (60ml), the organic phase was combined, and 35% HCl / ethanol solution (12ml) was added , a large amount of solids were precipitated, the mixture was stirred at room temperature for 1 h, filtered, the filter cake was collected, and vacuum-dried at 45° C. for 16 h to obtain 5.1 g of white solids with a yield of 75.7%. 1 HNMR (DMSO- d6 , 400MHz) δ 12.97(brs, 1H), 11.46(br,1H), 8.49(s, 1H), 7.50(s, 1H), 6.94(s, 1H), 5.32(m, 1...
Embodiment 2
[0034] Add N-methyl-N-((3R,4R)-4-methylpiperidin-3-yl)-7H-pyrrolo[2,3-d]pyrimidine-4 to a 250ml three-necked flask at 25°C -Amine hydrochloride (6.0g, 21.3mmol), add ethanol (60ml), stir to dissolve, then add acetic acid (2.2g, 32.0mmol), acetaldehyde (10.8g, 213mmol), then add NaBH in three batches 4 (1.6g, 42.6mmol), TLC (DCM:MeOH=10:1) after 1h reaction, the reaction was complete. Concentrate the reaction solution to dryness, add water (60ml) and EA (60ml) to the concentrate, stir and separate the layers, extract the water layer with EA (60ml) once more, combine the organic phases, add 35% HCl / ethanol solution (12ml) , a large amount of solids were precipitated, the mixture was stirred at room temperature for 1 h, filtered, the filter cake was collected, and vacuum-dried at 45°C for 16 h to obtain 4.5 g of white solids with a yield of 68.2%.
Embodiment 3
[0036] Add N-methyl-N-((3R,4R)-4-methylpiperidin-3-yl)-7H-pyrrolo[2,3-d]pyrimidine-4 to a 250ml three-necked flask at 25°C -Amine hydrochloride (6.0g, 21.3mmol), add methanol (60ml), stir to dissolve, then add formic acid (1.47g, 32.0mmol), acetaldehyde (10.8g, 213mmol), then add NaBH in three batches 4 (1.6g, 42.6mmol), TLC (DCM:MeOH=10:1) after 1h reaction, the reaction was complete. Concentrate the reaction solution to dryness, add water (60ml) and EA (60ml) to the concentrate, stir and separate the layers, extract the water layer with EA (60ml) once more, combine the organic phases, add 35% HCl / ethanol solution (12ml) , a large amount of solids were precipitated, the mixture was stirred at room temperature for 1 h, filtered, the filter cake was collected, and vacuum-dried at 45°C for 16 h to obtain 4.8 g of white solids with a yield of 72.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com