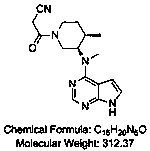
Preparation process of Xeljanz intermediate
A technology of tofacitinib and preparation process, which is applied in the direction of organic chemistry, can solve the problems of decreased purity, low purity, and decreased purity of intermediates, and achieve the effect of ensuring purity and reducing content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
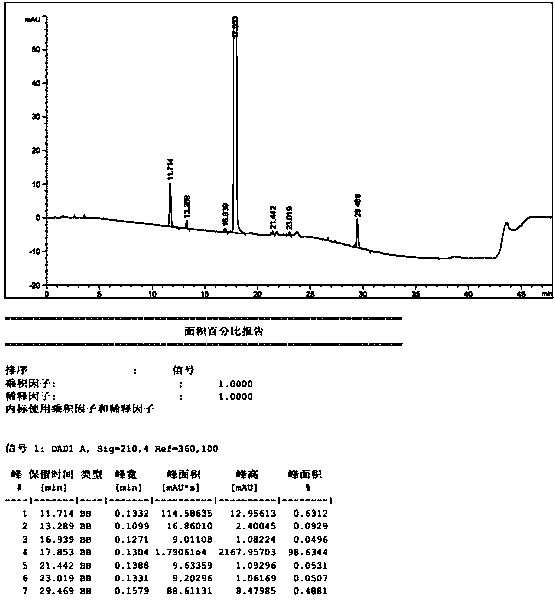
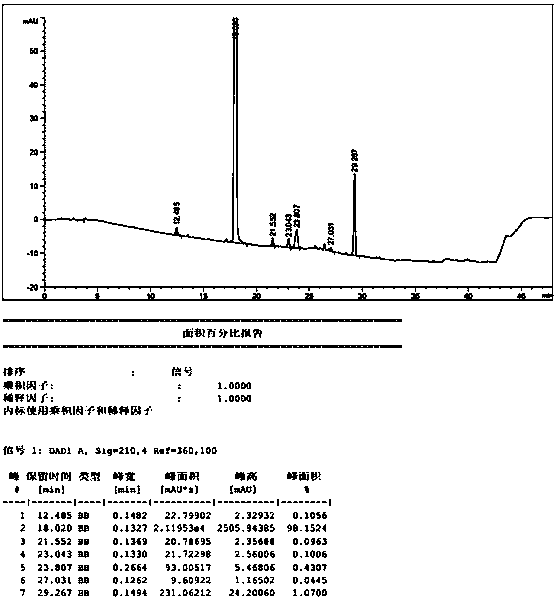
[0042] Add ethyl cyanoacetate (73.8g, 0.64mol) into 200mL n-butanol, stir and cool to 20°C, slowly add DBU (25g, 0.16mol) dropwise, and add N-methyl-N-( (3R,4R)-4-methylpiperidin-3-yl)-7H-pyrrolo[2,3-D]pyrimidin-4-amine (40g, 0.16mol), completed, heated to 35°C, reacted for 15h . Add 200mL of water dropwise into the reaction system, stir for 0.5h, and filter to obtain a white solid, which is washed with 100mL of ethanol / water (1:1) to obtain 47.5g of a white solid with a yield of 93.3% and a purity of 98.63%. The related substance A was not detected. out.
Embodiment 2
[0044] Add ethyl cyanoacetate (3.68kg, 32.5mol) into 10L n-butanol, stir and cool to 20°C, slowly add DBU (1.25kg, 8.2mol) dropwise, add N-methyl-N- ((3R,4R)-4-methylpiperidin-3-yl)-7H-pyrrolo[2,3-D]pyrimidin-4-amine (2.0kg, 8.2mol), completed, heated to 35°C, Reaction 15h. Add 10L of water dropwise into the reaction system, stir for 0.5h, and filter to obtain a white solid, which is washed with 2L of ethanol / water (1:1) to obtain 2.3kg of white solid with a yield of 90.2% and a purity of 98.53%. The content of related substance A is 0.05%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com