Preparation method of tofacitinib citrate
A technology of tofacitinib and citric acid, applied in the field of preparation of tofacitinib citrate, can solve the problems of unsuitability for industrialized production, complicated post-processing, harsh synthesis conditions and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
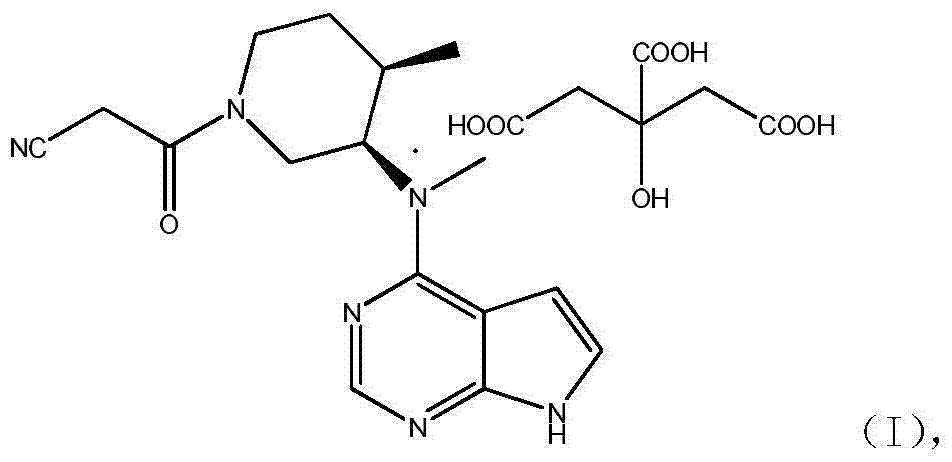
[0043] The third aspect of the present invention provides a preparation method of tofacitinib citrate crystal form A, which includes: using a nitrile solvent or a mixed solvent of a nitrile solvent and water and any solid form of the tofacitinib citrate method Mix Tini, heat to dissolve, cool down to precipitate crystals, and collect crystals.
[0044] The tofacitinib citrate of the present invention can be prepared by any existing disclosed technology, such as the method disclosed in Chinese patent CN 2002823587, and the tofacitinib citrate can be in any solid form, such as The solid tofacitinib citrate prepared by the prior art or the first aspect of the present invention or the amorphous tofacitinib citrate prepared by the second aspect of the present invention.
[0045] When the preparation method of the present invention dissolves tofacitinib citrate with a solvent, the dissolution methods such as stirring, heating, reflux and stirring can be used.
[0046] To obtain a s...
specific Embodiment approach
[0059] The embodiment of the present invention discloses a method for preparing tofacitinib citrate (I). Those skilled in the art can refer to the content of this article to appropriately improve the process parameters to achieve. In particular, it should be pointed out that all similar replacements and modifications are obvious to those skilled in the art, and they are all considered to be included in the present invention. The method of the present invention has been described through preferred embodiments, and relevant personnel can obviously make changes or appropriate changes and combinations to the method described herein without departing from the content, spirit and scope of the present invention to realize and apply the technology of the present invention .
[0060] In order to further understand the present invention, the present invention will be described in detail below in conjunction with examples.
Embodiment 1
[0061] Embodiment 1: the synthesis of tofacitinib citrate
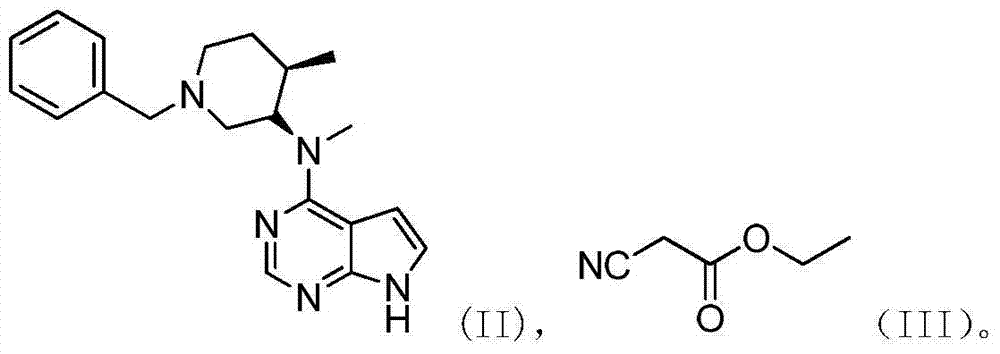
[0062] To the reaction vessel was added 10% palladium hydroxide on carbon (0.56g), methanol (20ml), N-methyl-N-{(3R,4R)-1-benzyl-4-methylpiperidine-3- Base}-7H-pyrrole[2,3-d]pyrimidin-4-amine (2.8g) and acetic acid (0.5g), first replaced by nitrogen, then hydrogen (1atm), reacted at 60°C for about 6 hours, and the reaction After completion, directly put DBU (3.8g) and ethyl cyanoacetate (3.7g), and react at room temperature for 6 hours to detect N-methyl-N-{(3R,4R)-1-benzyl-4-methylguanidine The ultraviolet fluorescence point of pyridin-3-yl}-7H-pyrrole[2,3-d]pyrimidin-4-amine disappears, and the treatment is directly put into citric acid (3.5g) and water (18ml) to stir and crystallize at room temperature, and keep warm for 2h After filtering, drying to obtain tofacitinib citrate (total yield 85%), the peak area of high performance liquid phase HPLC is 99.68%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com