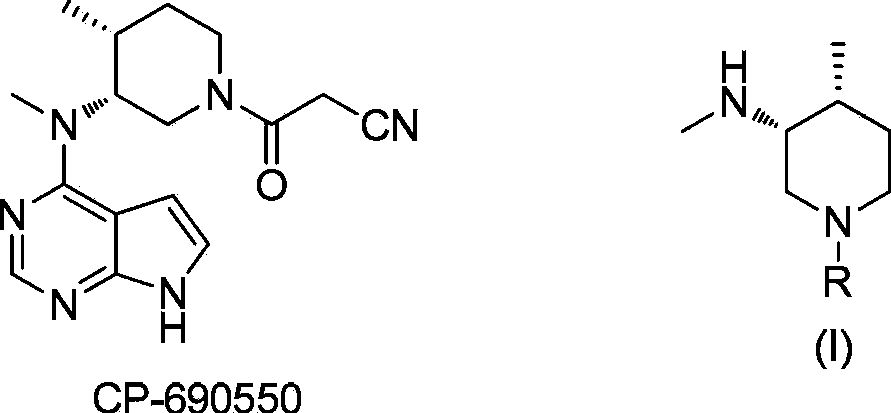
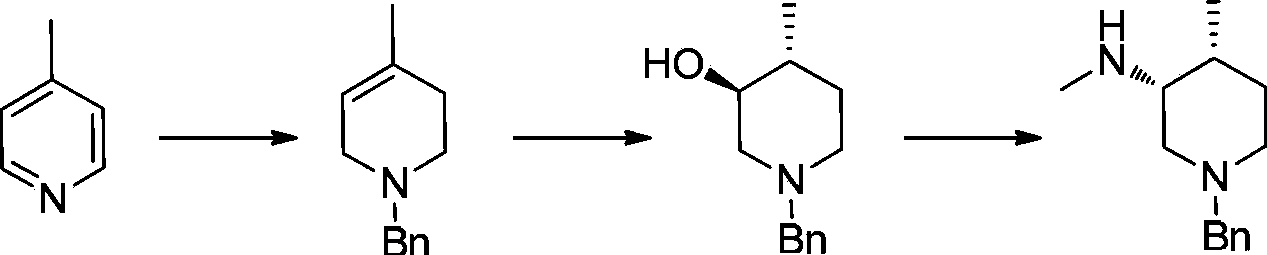
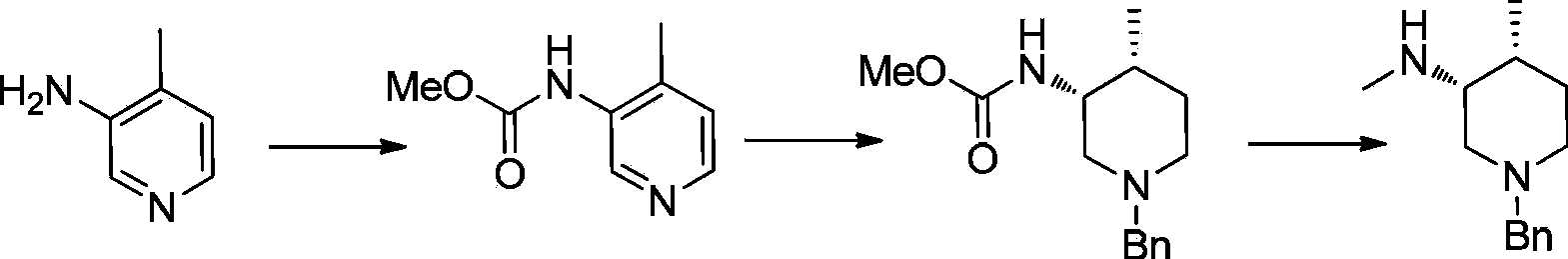
Asymmetric synthesis method of nitrogen protected (3R,4R)-3-methylamino-4-methylpiperidine, and relevant intermediate and raw material preparation method
A synthetic method, the technology of methylpiperidine, applied in the field of preparation of pharmaceutical intermediates, can solve the problems of expensive, long reaction steps, etc., and achieve the effect of simple route, cheap raw materials, flexible and convenient selection of synthetic methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Preparation of 1-benzyl-4-methyl-3-oxopiperidine-4-carboxylic acid methyl ester (1-2)
[0038]
[0039]Under nitrogen protection, add 1-benzyl-3-oxopiperidine-4-carboxylic acid methyl ester (1-1) (Shanghai Junyi Pharmaceutical Technology Co., Ltd.) 5.0g and potassium carbonate 8.4g into 100mL acetone , and then 5.8 g of methyl iodide was slowly added, and the reaction mixture was heated to 80° C. and refluxed for 12 hours. After the reaction was completed and cooled to room temperature, the solid was removed by filtration, and the solvent was evaporated under reduced pressure. The residue was dissolved in 50 mL of ethyl acetate and 50 mL of water, and the organic phase was separated after layering. The organic phase was washed with salt water (50ml*2 times), dried over anhydrous sodium sulfate, filtered, and spin-dried to obtain a light yellow viscous liquid 1-benzyl-4-methyl-3-oxopiperidine-4- 4.5 g of methyl carboxylate (1-2), yield: 85.0%. 1 H NMR (40...
Embodiment 2
[0040] Example 2: Preparation of 1-benzyl-4-methyl-piperidin-3-one (2-1)
[0041]
[0042] Add 10.0 g of 1-benzyl-4-methyl-3-oxopiperidine-4-carboxylic acid methyl ester (1-2) into 50 mL of 6 mol / L hydrochloric acid, and heat the reaction solution to 100°C and reflux for 5 Hour. After the reaction was completed, the temperature was lowered to room temperature, the solvent was distilled off under reduced pressure, the residue was dissolved in 50 mL of water, and the pH was adjusted to 10 with 2 mol / L aqueous sodium hydroxide solution, extracted three times by adding ethyl acetate, and the combined organic phase was washed with a small amount of salt water. Dry over anhydrous sodium sulfate and spin dry the organic solvent to obtain 6.8 g of yellow viscous liquid 1-benzyl-4-methyl-piperidin-3-one (2-1), yield: 87.5%. 1 H NMR (400MHz, CDCl 3 )δ7.30–7.25(m,5H),3.54(s,2H),3.20(d,J=12Hz,1H),2.89(m,1H),2.75(d,J=12Hz,1H),2.41– 2.31(m,2H),2.00(m,1H),1.62–1.60(m,1H),1.05(s,3H).ESI...
Embodiment 3
[0043] Example 3: Preparation of 1-tert-butoxycarbonyl-4-methyl-piperidin-3-one (3-1)
[0044]
[0045] Dissolve 5.0 g of 1-benzyl-4-methylpiperidin-3-one (2-1) in 50 mL of tetrahydrofuran, and add 0.5 g of Pd / C (5%) and 5.9 g of di-tert-butyl dicarbonate ester, and the reaction mixture was hydrogenated at room temperature and pressure for 12 hours. After the reaction was completed, filter with a sand core funnel lined with diatomaceous earth, and spin the filtrate to obtain 5.2 g of light yellow viscous liquid 1-tert-butoxycarbonyl-4-methylpiperidin-3-one (3-1). Yield: 99.9%. 1 H NMR (400MHz, CDCl 3 )δ3.91–3.99(m,2H),3.39(m,1H),2.48–2.43(m,1H),2.12–2.07(m,1H),1.64–1.50(m,2H),1.64(s, 9H),1.13(d,J=5.3Hz,3H).ESI-MS:m / z=214(M + +1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com