Patents
Literature
43 results about "1-Phenylethylamine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
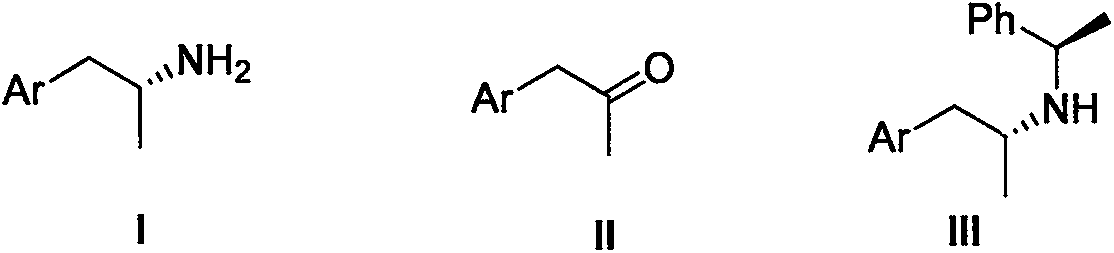
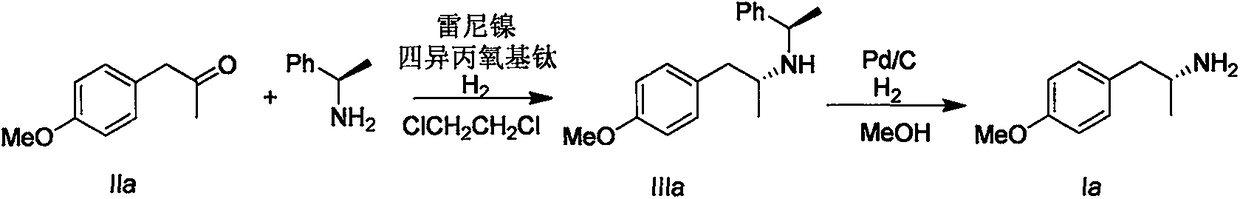
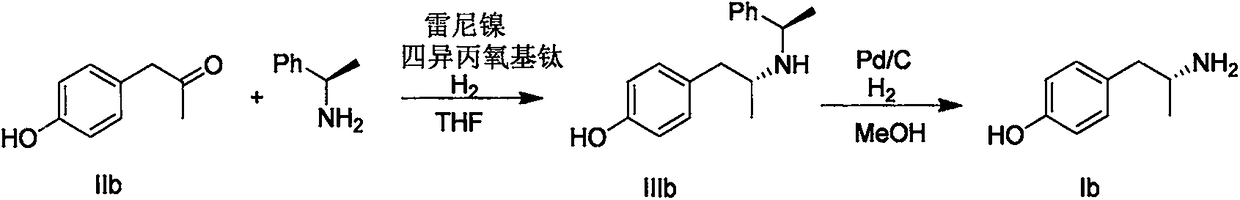
1-Phenylethylamine is the organic compound with the formula C₆H₅CH(NH₂)CH₃. Classified as a monoamine, this colorless liquid is often used in chiral resolutions. Like benzylamine, it is highly basic and forms stable ammonium salts and imines.
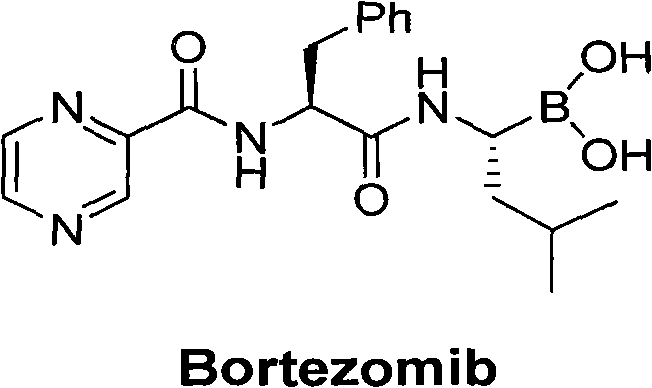
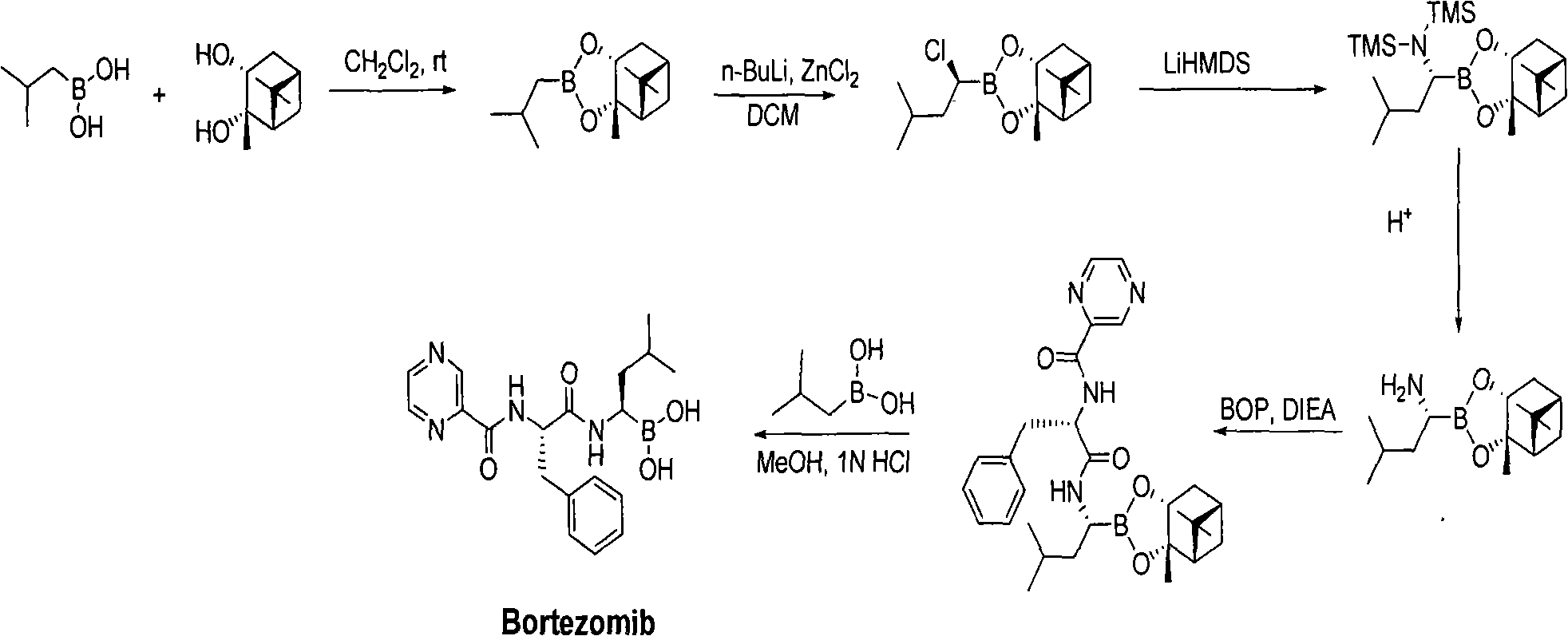
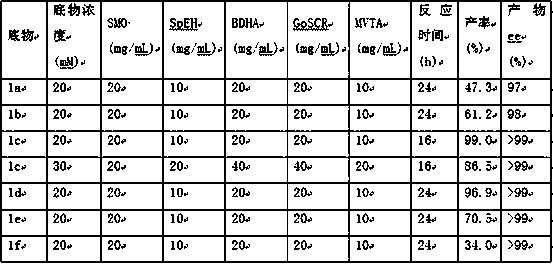
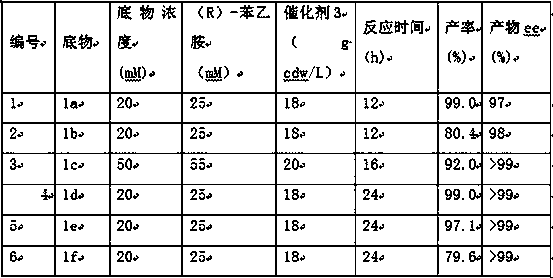
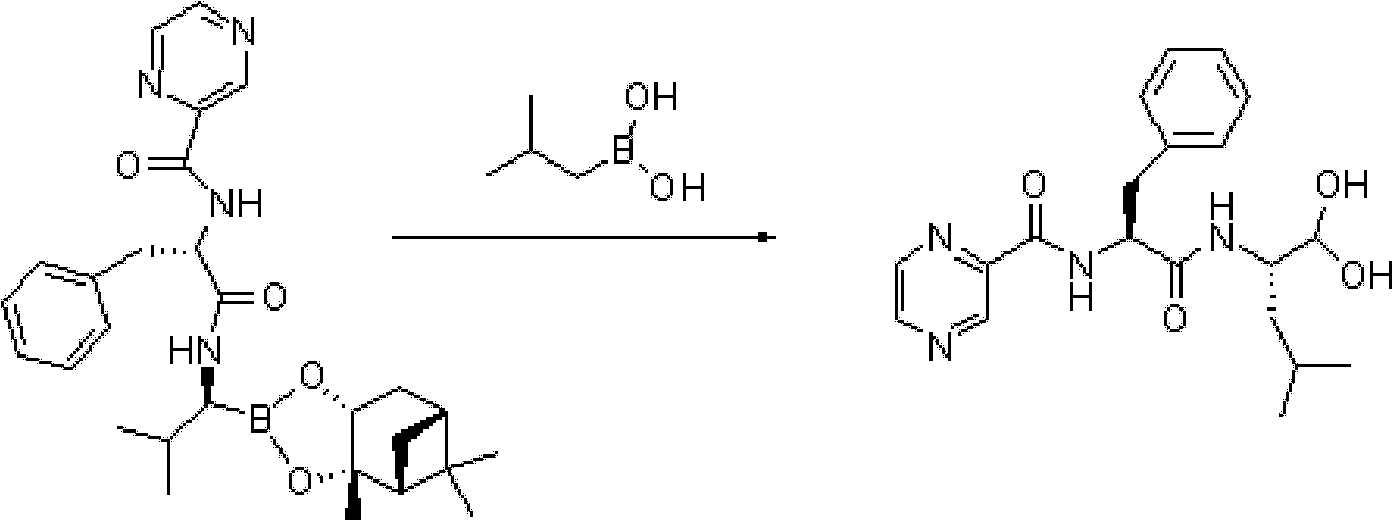
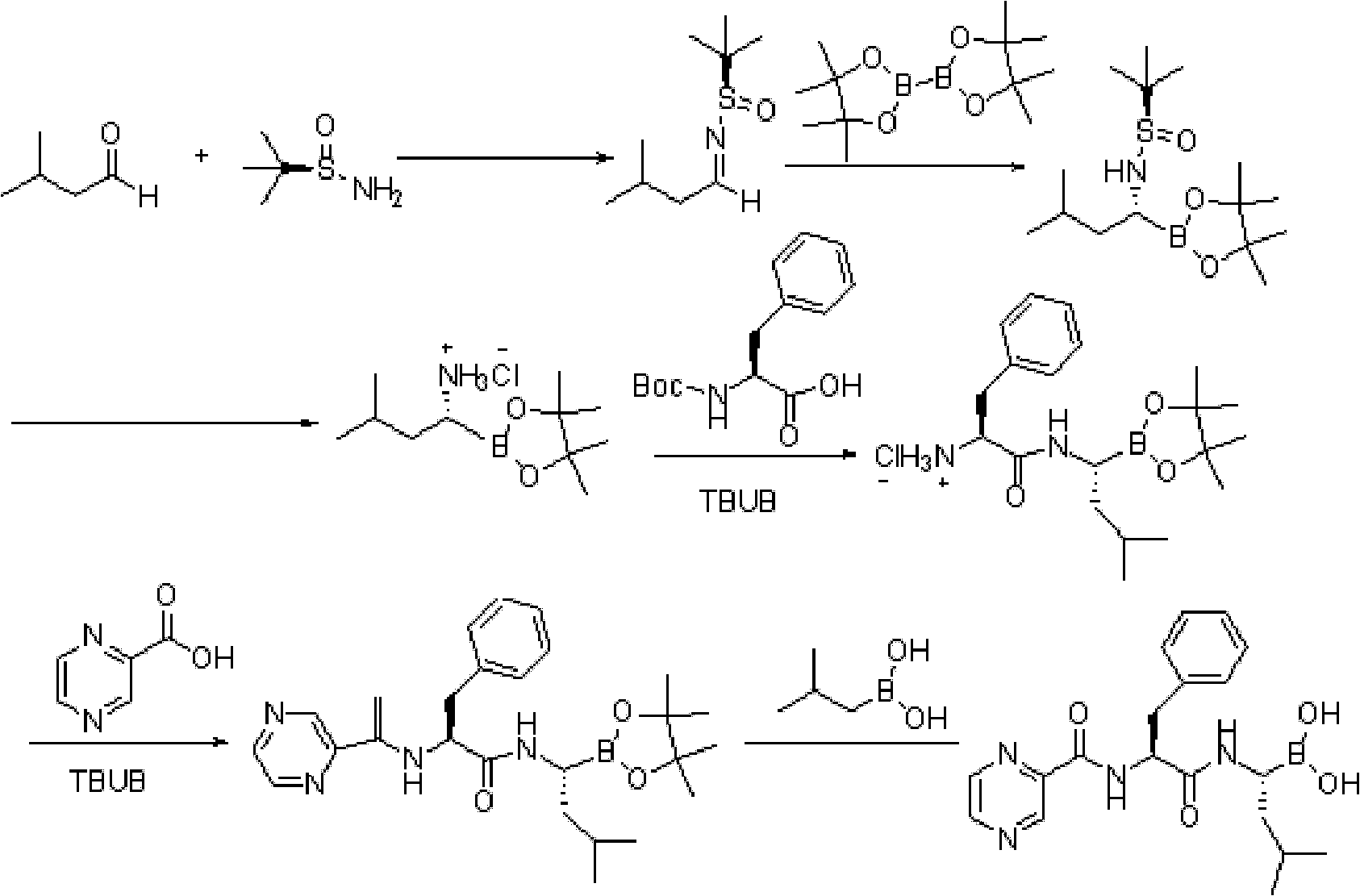
Method for synthesizing bortezomib
The invention belongs to the field of synthesizing medicaments, and discloses a method for synthesizing bortezomib. In the method, 3-methyl butyraldehyde and R-(+)-1-phenylethylamine are used as initiative materials, and the [(1R)-3-methyl-1-[[(2S)-1-oxygen-3-phenyl-2[(pyrazine formyl) amino] propyl]amino]butyl]-boric acid is obtained by condensation, selective boric acid ester addition, hydrogenation deprotection, chiral condensation with L-phenylalanine, condensation with 2-carboxyl-piperazine and boric acidification. The synthesis method has the advantages of readily available raw materials, higher yield of the whole reaction route, mild reaction conditions, easy operation, lower production cost and the suitability for industrialized production.
Owner:CHANGZHOU YABANG PHARMA
Method for preparing (3R)-(-)-3-(2- acetamino)-5-methylhexanol
InactiveCN102898320AEmission reductionReduce manufacturing costOrganic compound preparationCarboxylic acid amides preparation1-phenylethanamineAlcohol
The invention provides a method for preparing an antiepileptic medicament pregabalin intermediate, i.e., (3R)-(-)-3-(2-acetamino)-5-methylhexanol. The method comprises the following steps of: salifying a (+ / -)-3-(2-acetamino)-5-methylhexanol racemic body serving as a raw material and (S)-(-)-1-phenylethylamine in a mixed solvent of alcohol and halogenated hydrocarbon, precipitating unnecessary S-shaped chiral isomer salts out, filtering, concentrating a filtrate, dissolving into water, and reacting by using an acid to obtain (3R)-(-)-3-(2- acetamino)-5-methylhexanol of which the e.e. value is 80-90 percent; and refining to obtain a (3R)-(-)-3-(2-acetamino)-5-methylhexanol product of which the e.e. value is 98-99 percent. The method has the advantages of high chiral monomer purity, wide reaction temperature range, convenience in operating and suitability for industrial production. The invention further provides a method for preparing a racemate, i.e., (+ / -)-3-(2-acetamino)-5-methylhexanol. The racemic body can be taken as a raw material for repeated use, so that the consumption of raw materials is lowered, environmental protection is promoted, and the method has high application value.
Owner:AURISCO PHARMACEUTICAL CO LTD
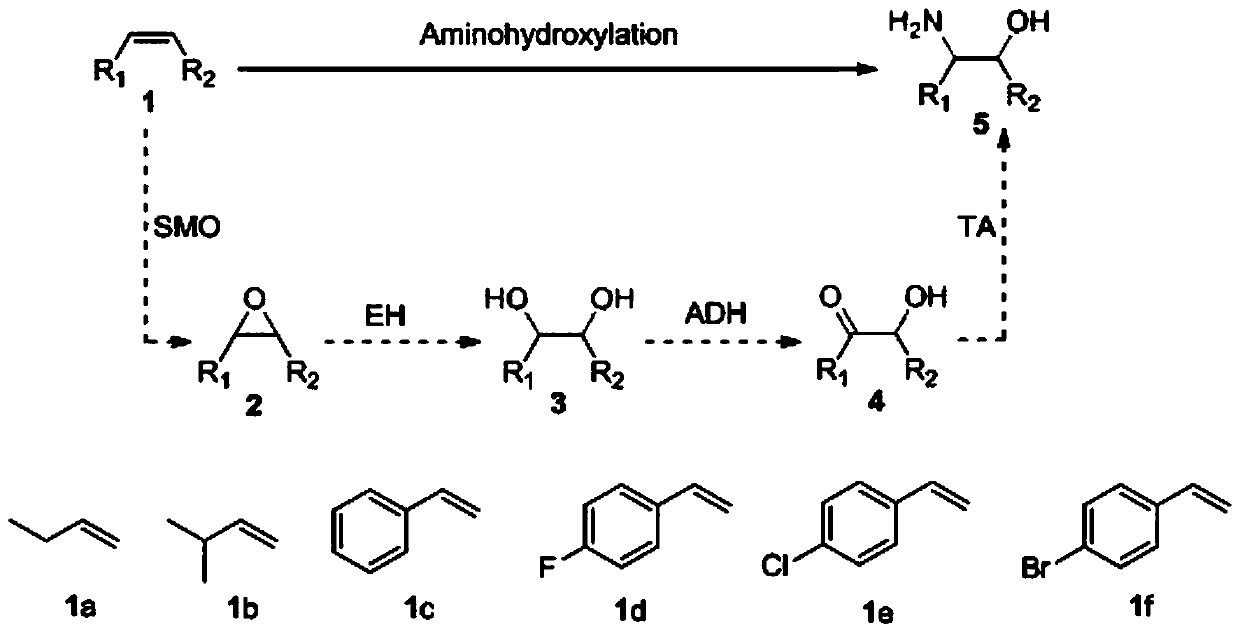
Method for preparing chiral beta-amino alcohol by asymmetric amine hydrogxylation of olefin through cascade biocatalysis
InactiveCN110172484AAvoid lostAchieve recyclingMicroorganism based processesFermentationEscherichia coliEpoxide metabolism
The invention belongs to the technical field of biology, and provides a method for preparing chiral beta-amino alcohol by asymmetric amine hydroxylation of an olefin compound through cascade biocatalysis. Olefin monooxygenase, epoxide hydrolase, alcohol dehydrogenase and transaminase are taken as biocatalysts; olefin is used as a substrate and a reaction is performed in a phosphate buffer solutionto synthesize the chiral beta-amino alcohol. The conversion rate reaches up to 99% and the ee value is 97-99%. The olefin monooxygenase / alcohol dehydrogenase (ADH) / (R)-(+)-1 phenylethylamine (R-MBA)in a cascade biocatalytic system drives the reaction to be carried out towards a direction beneficial for product generation, and a cofactor NAD<+> is regenerated. The whole-cell cascade biocatalysisis realized through using engineering recombinant escherichia coli cells, and the chiral beta-amino alcohol is obtained. The method is used for converting the cheap olefin compound into various different chiral beta-amino alcohols; the catalytic efficiency is high, reaction conditions are mild, a reaction process is simple, the energy consumption is low, and the method is in line with the principle of green chemistry.
Owner:TAIYUAN UNIV OF TECH
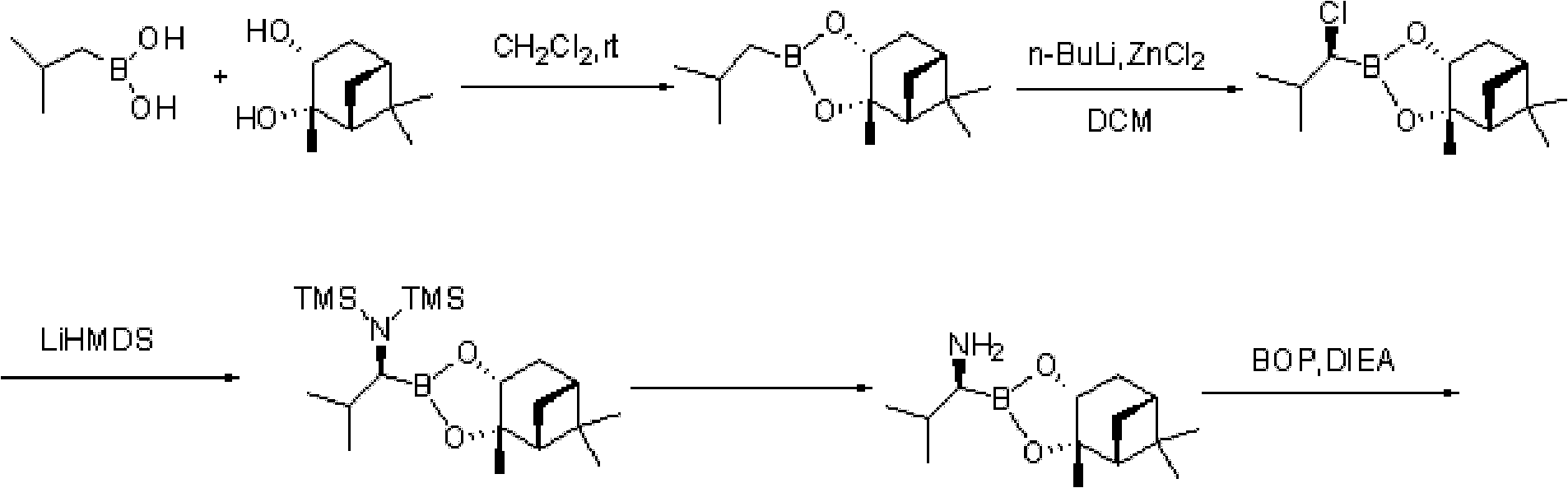
Method for preparing intermediate used for synthesizing bortezomib
ActiveCN103044467ARaw materials are cheap and easy to getEasy to operateGroup 3/13 element organic compoundsBoronic acidMethyl group
The invention discloses a method for preparing an intermediate used for synthesizing bortezomib, comprising the following steps of: carrying out an addition reaction on 3-methyl butyraldehyde and bis(pinacolaton)diboron, then carrying out sulfonylation or halogenating reaction, carrying out an amination reaction with R-(+)-1-phenylethylamine, carrying out catalytic hydrogenation a debenzylation reaction, and finally carrying out enantiomer resolution. Compared with the prior art, the preparation method of the R-(1-amino-3-methyl) butyl boronic acid pinacol cyclic ester intermediate, provided by the invention, has the advantages that raw materials are cheap and easy to get, operation is easy, reaction conditions are mild and optical purity is high, and especially post-processing is simple and the high-optical-purity bortezomib can be easily obtained without column chromatography separation when the intermediate prepared by the invention is used for synthesizing the bortezomib, so that industrial production requirement of the bortezomib is met and the preparation method provided by the invention has obvious effect and economic practicability.
Owner:重庆瑞泊莱制药有限公司
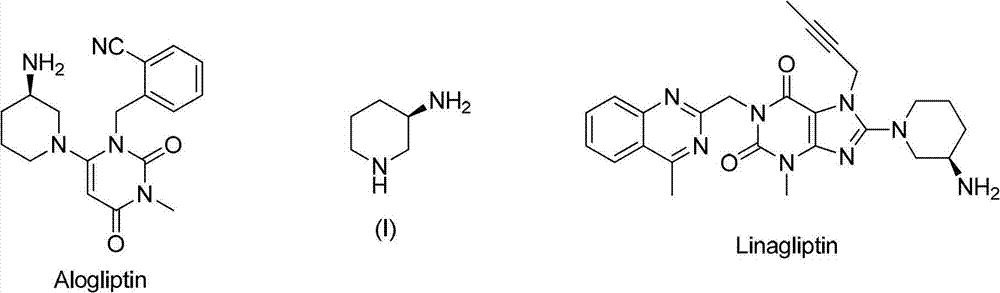
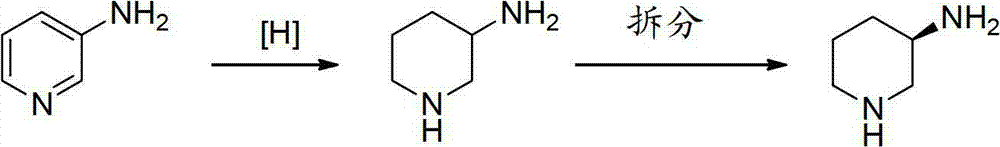
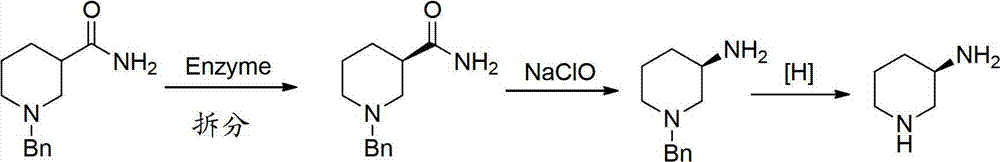
Asymmetric syntheses method and correlated intermediate of (R)-3-aminopiperidine (I)
ActiveCN103588699AHigh enantiopurityEasy to operateOrganic chemistryBulk chemical production1-phenylethanamineSynthesis methods
The invention relates to an asymmetric syntheses method of (R)-3-aminopiperidine (I). The method comprises: reducing a formula (III) compound to obtain a formula (II) compound, and then removing a chiral prosthetic group and an amino protective group from the formula (II) compound to obtain (R)-3-aminopiperidine (I), wherein R is the amino protective group, and specially is C1-4 alkoxycarbonyl or benzyl removable by hydrolysis or hydrogenation. Preferably, the formula (III) compound is obtained by performing a dehydration reaction on 3-piperidone and a chiral amine (R)-1-phenylethylamine. The invention also relates to a new compound (II). The asymmetric syntheses method of (R)-3-aminopiperidine (I) is reasonable in technology and concise in route, the needed product with relatively high ee value is obtained by utilizing chiral induction, the raw material is cheap and does not need resolving, no waste isomer is discharged, and the asymmetric synthesis method is applicable to large-scale industrial production.
Owner:SHANGHAI PUYI CHEM CO LTD
Metal organic polymer-doped nano-silver composite material sensor, and preparation method and application thereof
InactiveCN106226375AEasy to prepareReduce energy consumptionMaterial electrochemical variables1-phenylethanamineEnantiomer
The invention discloses a metal organic polymer {[CuL]2(H2O)}n-doped nano-silver composite material sensor, and a preparation method and application thereof. The preparation method comprises the following steps: (1) taking alkaline reducing Schiff alkali ligands H2L, nano-silver solution and CuAc2 solution as raw materials, and performing ultrasonic treatment to obtain a metal organic polymer-doped nano-silver composite material; (2) modifying an electrochemical chiral working electrode by using the composite material to obtain a chiral sensor; (3) detecting the content of (R)-(+)-1-phenylethylamine and (S)-(-)-1-phenylethylamine enantiomer by adopting a three-electrode system. The preparation method for the composite material and the chiral sensor is simple and convenient and is easy to operate; the technical effect of detecting the content of (R)-(+)-1-phenylethylamine and (S)-(-)-1-phenylethylamine enantiomer is remarkable.
Owner:UNIV OF JINAN
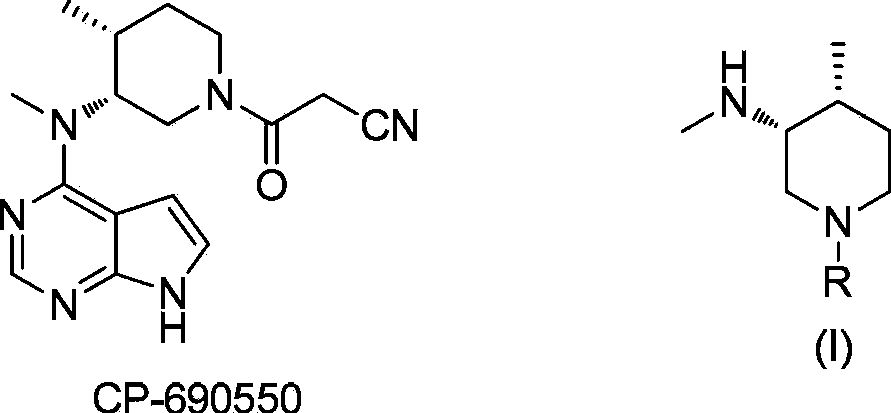
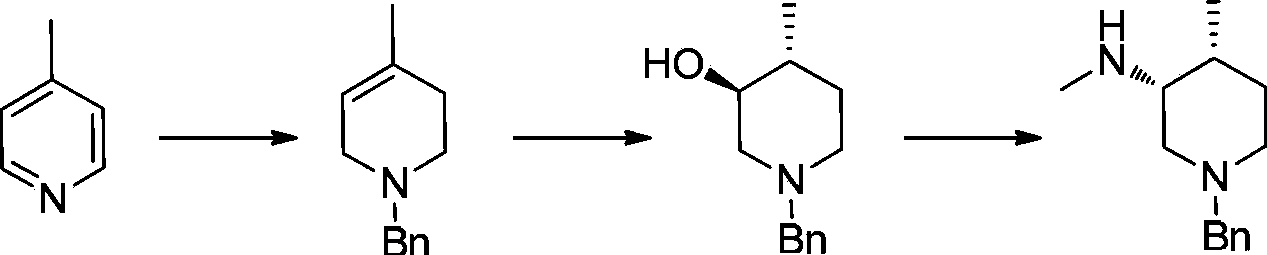
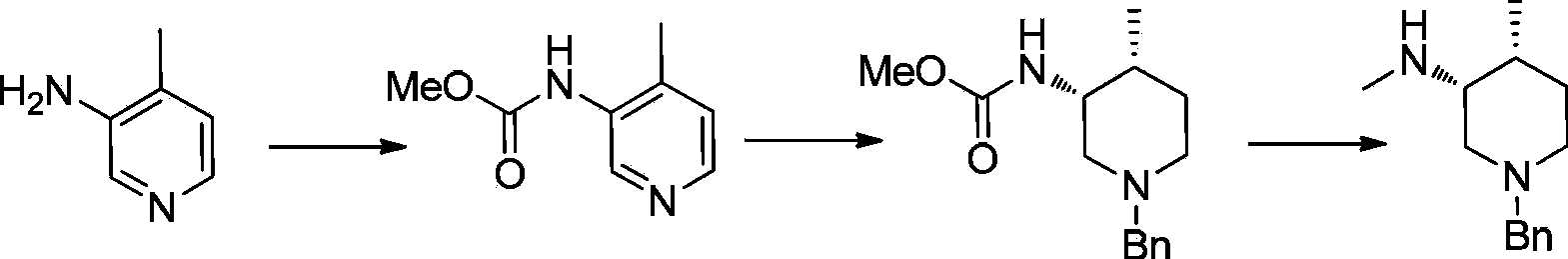
Asymmetric synthesis method of nitrogen protected (3R,4R)-3-methylamino-4-methylpiperidine, and relevant intermediate and raw material preparation method
ActiveCN103896826ANo emissionsSave raw materialsOrganic chemistryBulk chemical production4-methylpiperidineSynthesis methods
The invention relates to a preparation method of nitrogen protected (3R,4R)-3-methylamino-4-methylpiperidine (I). The method comprises the following steps: carrying out a reductive amination reaction on a compound of formula (III) and (R)-1-phenylethylamine to obtain a compound of formula (II), removing chiral prosthetic groups from the compound of formula (II), and adding a methyl group to the amino group of the compound of formula (II) in order to obtain nitrogen protected (3R,4R)-3-methylamino-4-methylpiperidine (I), wherein R in each of the formula (I), the formula (II) and the formula (III) is an amino protection group or hydrogen, and the amino protection group can be C1-4 alkoxycarbonyl, benzyloxycarbonyl or benzyl groups which can be removed through hydrolysis or hydrogenation. The asymmetric synthesis method of nitrogen protected (3R,4R)-3-methylamino-4-methylpiperidine (I) has the advantages of reasonable technology, concise route, obtaining of the required product in a high ee value manner by constructing two chiral centers through chiral induced one-step reductive amination, cheap raw materials, and no waste isomer emission, and is suitable for large-scale industrialized production.
Owner:SHANGHAI PUYI CHEM CO LTD
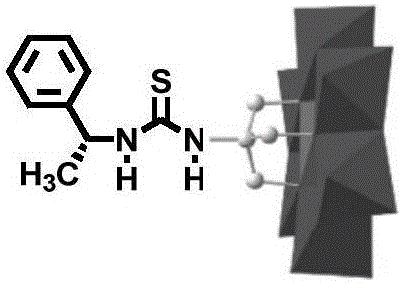
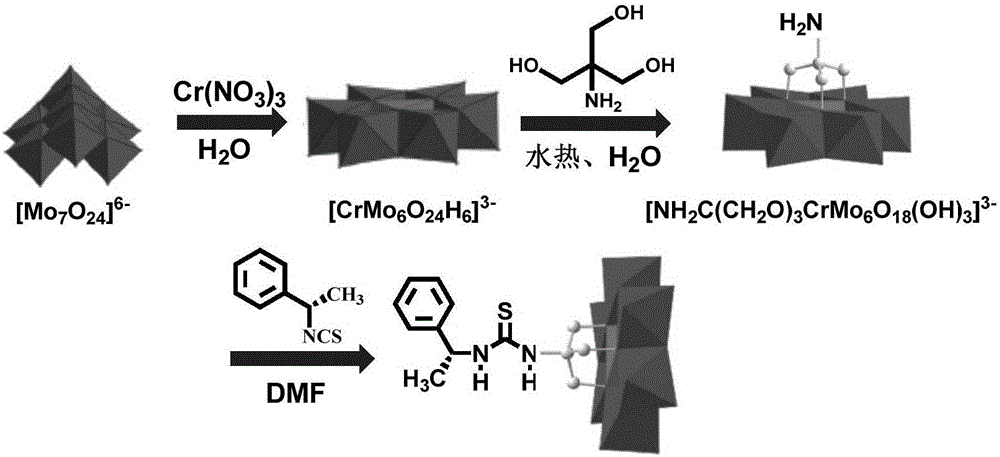
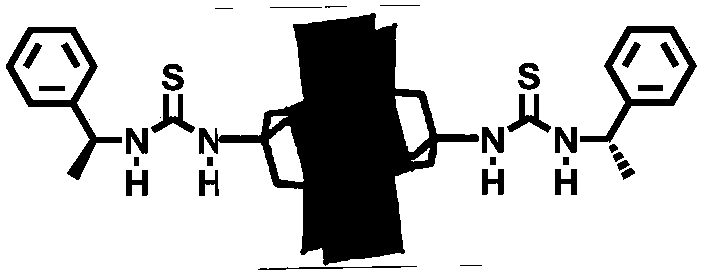
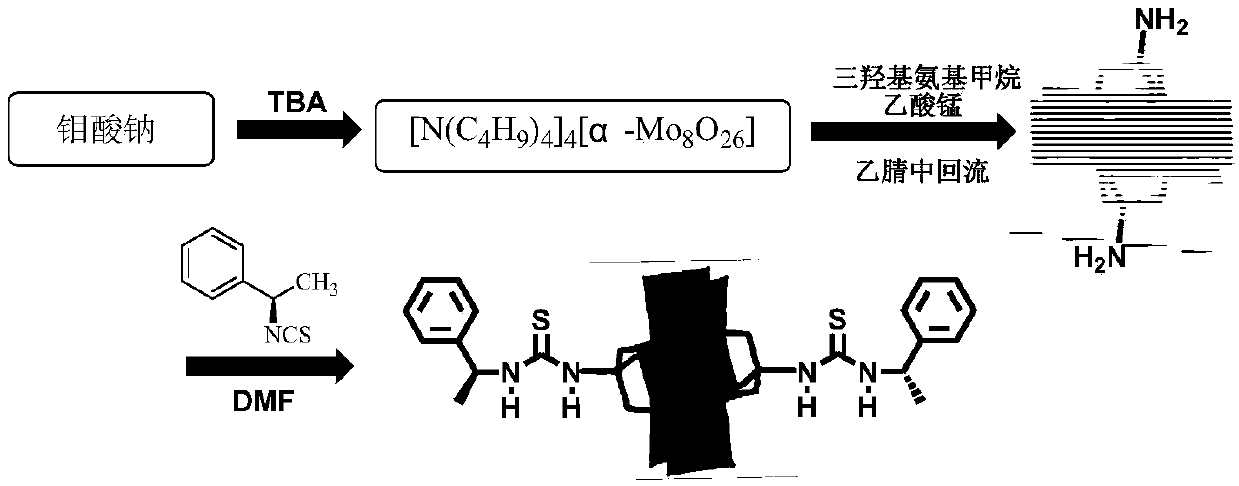
(S)-1-(1-phenethyl) thiourea-modified Cr-Anderson heteropolyacid catalyst, and preparation method and application thereof
ActiveCN105854941AHigh stereoselectivityNoveltyPreparation by oxidation reactionsOrganic compound preparationThioureaIsothiocyanic acid
The invention discloses an (S)-1-(1-phenethyl) thiourea-modified Cr-Anderson heteropolyacid catalyst, and a preparation method and an application thereof. The method comprises the following steps: firstly, reacting ammonium molybdate and chromic nitrate to generate Cr-Anderson heteropolyacid (NH4)3[Cr(OH)6Mo6O18]; carrying out hydrothermal reaction on the Cr-Anderson heteropolyacid and tris(hydroxymethyl)aminomethane in a hydrothermal kettle to obtain an organic unilateral amino-modified polyoxometallate; and with (S)-(+)-1-phenylethylamine as a raw material, synthesizing (R)-1-(1-phenethyl) isothiocyanic acid; and finally reacting (R)-1-(1-phenethyl) isothiocyanic acid and the organic unilateral amino-modified polyoxometallate to obtain the target heteropolyacid catalyst. The preparation method is simple; and the obtained heteropolyacid catalyst is applied to asymmetric dihydroxylation of olefin, is friendly to environment, can be recycled, has high enantioselectivity and high catalytic activity and is suitable for industrial production.
Owner:上海元革新材料科技有限公司
Process for the production of enantiomerically enriched N-acylazetidine-2-carboxylic acids
PCT No. PCT / GB97 / 01915 Sec. 371 Date Dec. 16, 1998 Sec. 102(e) Date Dec. 16, 1998 PCT Filed Jul. 15, 1997 PCT Pub. No. WO98 / 02417 PCT Pub. Date Jan. 22, 1998Process for obtaining an enantiomerically enriched N-acylazetine-2-carboxylic acid by selectine crystallization of a diastereoisomeric salt formed by relcting an enantiomer of the N-acylazetidine-2-carboxylic acid and an enantiomer of 1-phenylethylamine.
Owner:ASTRAZENECA AB
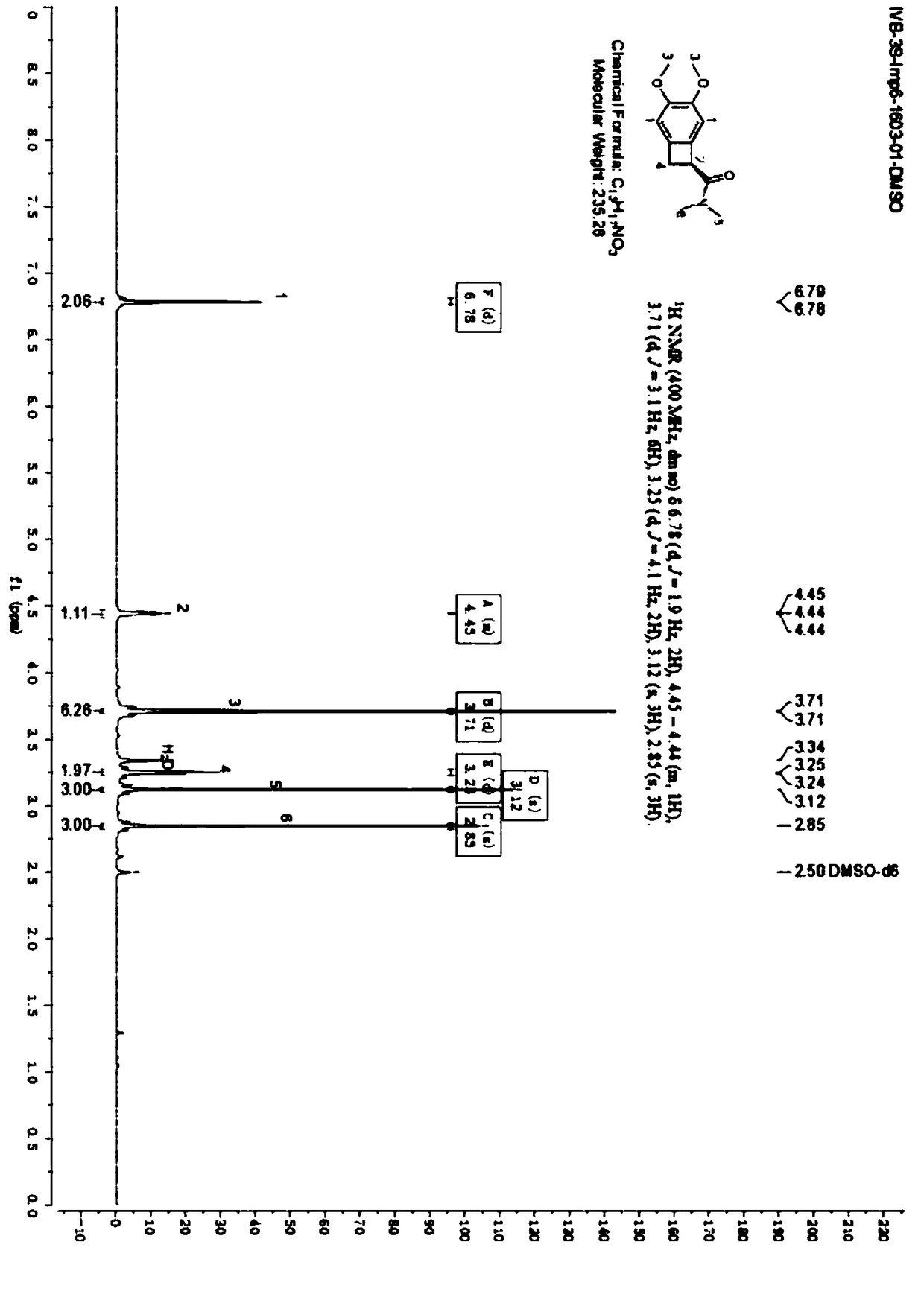
Method for synthesizing (1S)-4,5-dimethoxy-1-(carbonylaminomethyl)benzocyclobutane
ActiveCN108947800AHigh chiral purityEasy to recycleOrganic compound preparationCarboxylic acid amides preparation1-phenylethanamineOrganic acid
The invention relates to a method for synthesizing (1S)-4,5-dimethoxy-1-(carbonylaminomethyl)benzocyclobutane. The method provided by the invention does not need complicated high-cost column chromatographic methods for product purification or other special equipment, is good in operability, mild and safe in process, simple in reaction operation and beneficial for industrial production; and halogenated hydrocarbons are used as a solvent and are easy to recover and to use indiscriminately, and S-1-phenylethylamine is used as a resolving agent and is low in price and easy to recover, so production cost is greatly reduced. According to the invention, the synthesis is performed in organic acid, so racemization pollution is low, and environmentally friendliness is achieved; and the produced (1S)-4,5-dimethoxy-1-(carbonylaminomethyl)benzocyclobutane has few impurities and high chiral purity, wherein yield can reach 80-90% and HPLC purity is no less than 99%, so product quality is good.
Owner:安徽美诺华药物化学有限公司
Method for preparing S-4-methoxymandelic acid through splitting S-1-phenylethylamine
InactiveCN105130794ARaw materials are easy to getMild conditionsOrganic compound preparationOptically-active compound separation1-phenylethanamineAlcohol
The invention discloses a method for preparing S-4-methoxymandelic acid through splitting. The method concretely includes the steps that in alcohol solvent, racemic 4-methoxymandelic acid is used as a raw material, S-1-phenylethylamine is used as a splitting agent for reaction, then S-1-phenylethylamine salt of S-4-methoxymandelic acid is obtained through cooling, crystallizing and separating, and S-4-methoxymandelic acid is obtained through acid freeing after salt recrystallization is performed; after the solution containing the splitting agent is mixed, alcohol is removed through evaporation, after cooling, water and alkaline are added for freeing, and the splitting agent which is S-1-phenylethylamine is obtained through extracting, drying and concentrating. The method for preparing S-4-methoxymandelic acid through splitting has the advantages that conditions are mild, operation is easy, the product yield is good, optical purity is high, and the splitting agent can be recycled, and is quite suitable for preparing and producing S-4-methoxymandelic acid.
Owner:彭静
Improved synthetic method of (R)-1-aryl-2-propylamine
InactiveCN108250086AHigh yieldHigh selectivityOrganic compound preparationOrganic chemistry methodsPropylamineLewis acid catalysis
The invention provides an improved synthetic method of (R)-1-aryl-2-propylamine. The improved synthetic method comprises the following steps of: adopting 1-arylacetone as a raw material, adding (R)-1-phenylethylamine, carrying out reductive amination reaction with hydrogen under a common action of a Lewis acid additive and a transitional metal hydrogenation catalyst; carrying out Pd / C catalytic hydrogenation on an obtained intermediate to remove phenethyl on nitrogen and thus obtaining (R)-1-aryl-2-propylamine. The improved synthetic method provided by the invention has the beneficial effectsthat the operation is simple, the adopted Lewis acid additive is low in cost and easy in obtaining, the yield and the enantioselectivity of a product are high, and the application value for industrialsynthesis of (R)-1-aryl-2-propylamine is very high.
Owner:SUN YAT SEN UNIV
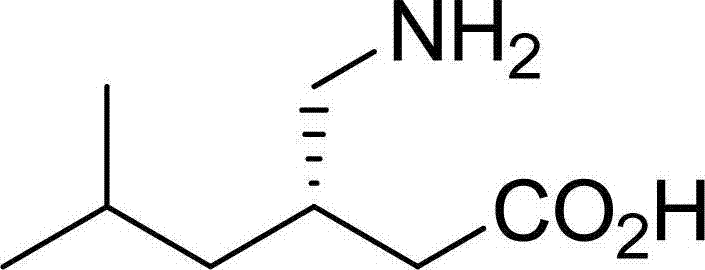
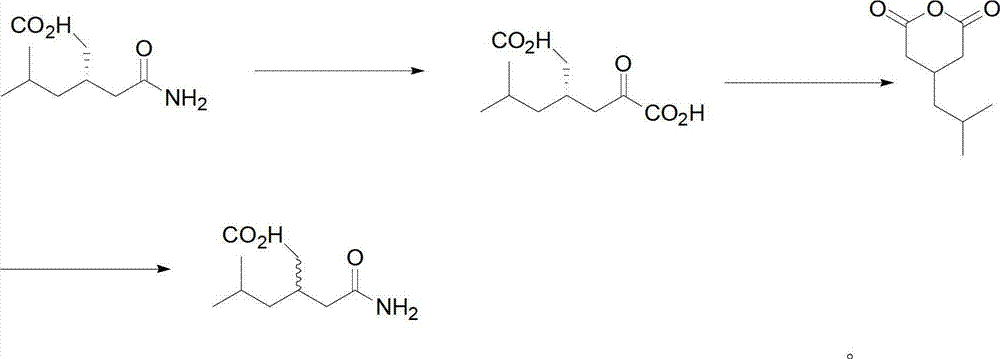
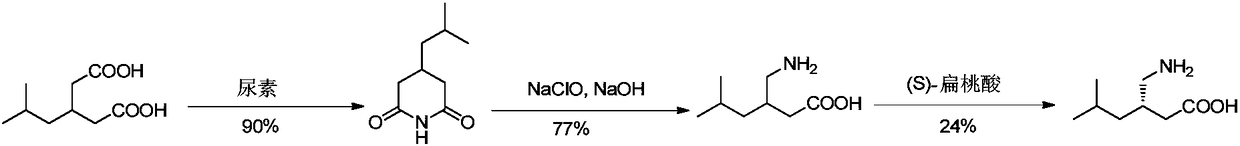
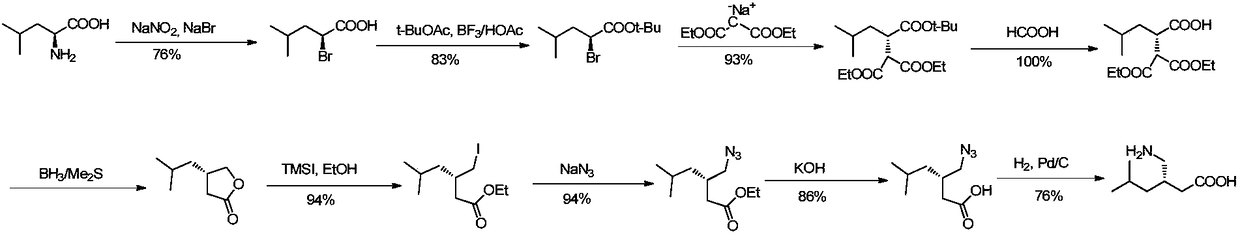
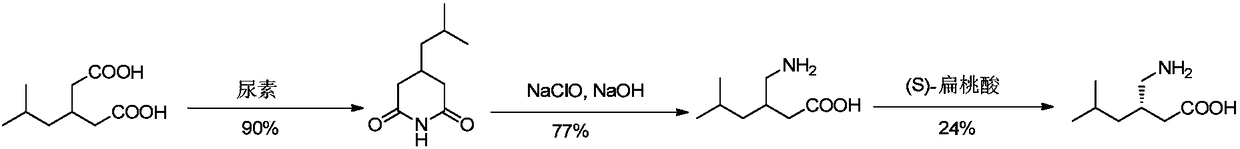
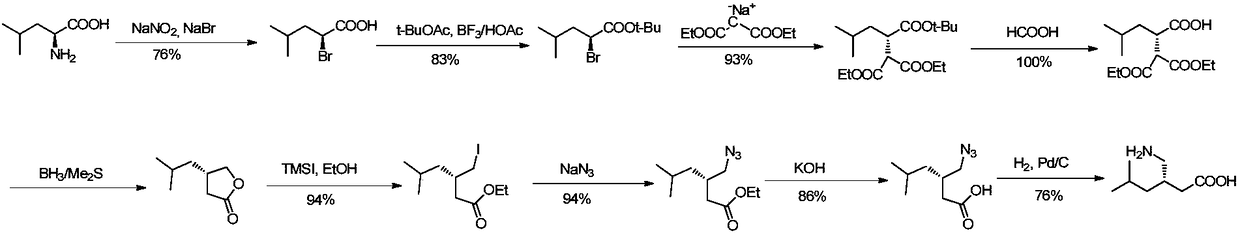
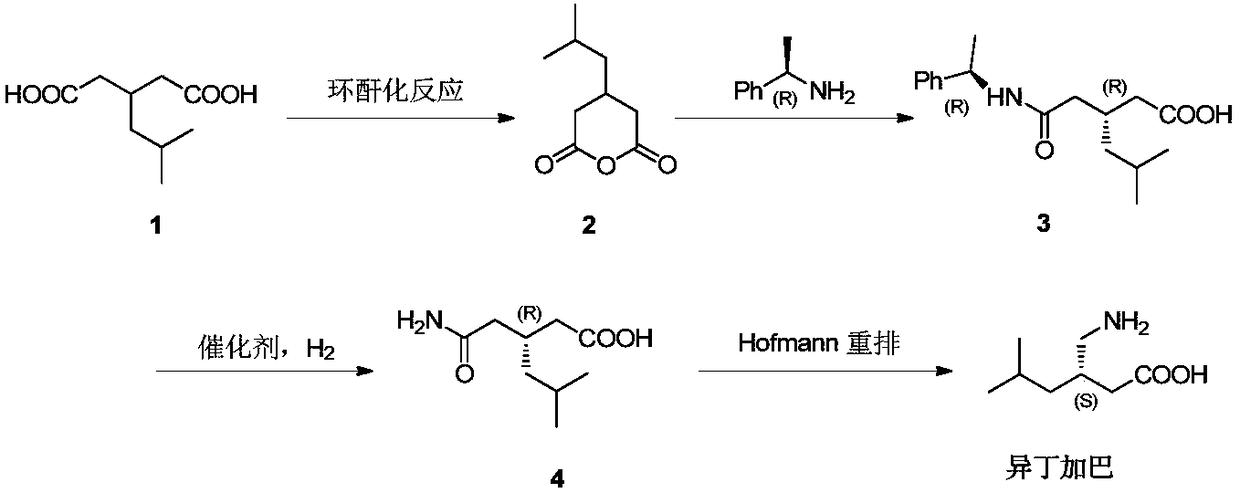
Method for asymmetric preparation of (S)-3-aminomethyl-5-methylcaproic acid
InactiveCN108456143AReduce usageRaw materials are cheap and easy to getOrganic compound preparationOrganic chemistry methodsHydrolysisReaction step
The invention discloses a method for asymmetric preparation of (S)-3-aminomethyl-5-methylcaproic acid. The method is characterized by comprising the following four synthesis steps: with 3-isobutylglutaric acid as a raw material, subjecting 3-isobutylglutaric acid and a nitrogen-containing reagent to a ring-closure reaction; subjecting a reaction product and (S)-(+)-1-phenylethylamine to asymmetricring opening; carrying out Huffman rearrangement; and then carrying out amide hydrolysis so as to obtain (S)-3-aminomethyl-5-methylcaproic acid. Compared with the prior art, the method provided by the invention has the advantages of usage of cheap and easily available raw materials, short reaction steps, mild reaction conditions, and no usage of reagents easily leading to poisoning and explosion;the overall yield of method is as high as 72%; the purity of the product pregabalin is greater than 99%, and an ee value is greater than 99%; and the method has good application prospects in industrial large-scale production.
Owner:SYNCOZYMES SHANGHAI
Preparation method of sitafloxacin side chain intermediate
InactiveCN104230790AHigh yieldImprove securityOrganic chemistryBulk chemical productionSide chainEthylamine
The invention relates to a preparation method of a sitafloxacin side chain intermediate. The preparation method comprises the following steps: synthesizing 4-methyl acetoacetate with 1,2-dibromoethane into a three-membered ring, synthesizing the three-membered ring with ammonia water into nitrogen heterocycle, synthesizing nitrogen heterocycle with R-1-phenyl ethylamine to generate chiral carbon, reducing through sodium borohydride, adding ditertbutyl dicarbonate to generate a protecting group, and finally performing carbonyl reduction to borane-dimethyl sulfide to obtain an intermediate. According to the preparation method, the atom utilization rate can be improved, the use of toxic reagents can be reduced, and the amplification of the technology can be facilitated.
Owner:SUZHOU NACHI BIOTECH CO LTD
Method for the racemization of etodolic acid
A method for the resolution of etodolic acid by crystallization of its salt with optically active 1-phenylethylamine and subsequent recovery of the (R,S) etodolic acid from the mother liquors of crystallization by racemization and crystallization is described.
Owner:CHEMI SPA
Method for the racemization of etodolic acid
A method for the resolution of etodolic acid by crystallization of its salt with optically active 1-phenylethylamine and subsequent recovery of the (R,S) etodolic acid from the mother liquors of crystallization by racemization and crystallization is described.
Owner:CHEMI SPA
Improved method used for preparing ambrisentan
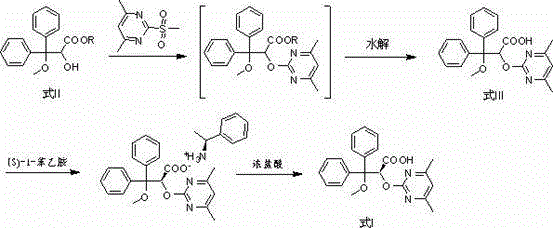
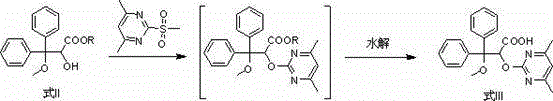
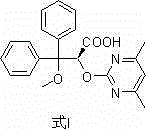
The invention belongs to the field of chemical synthesis, and discloses an improved method used for preparing ambrisentan. According to the improved method, 2-hydroxy-3-methoxy-3,3-diphenylpropionic ester and 4,6-dimethyl-2-methylsulfonylpyrimidine are subjected to condensation, and one-pot hydrolysis in tetrahydrofuran solvent so as to obtain ambrisentan racemate; and ambrisentan is obtained via resolution with (S)-1-phenylethylamine. The improved method is simple in operation, and high in yield, and is suitable for industrial production.
Owner:山东瑞银生物工程有限公司
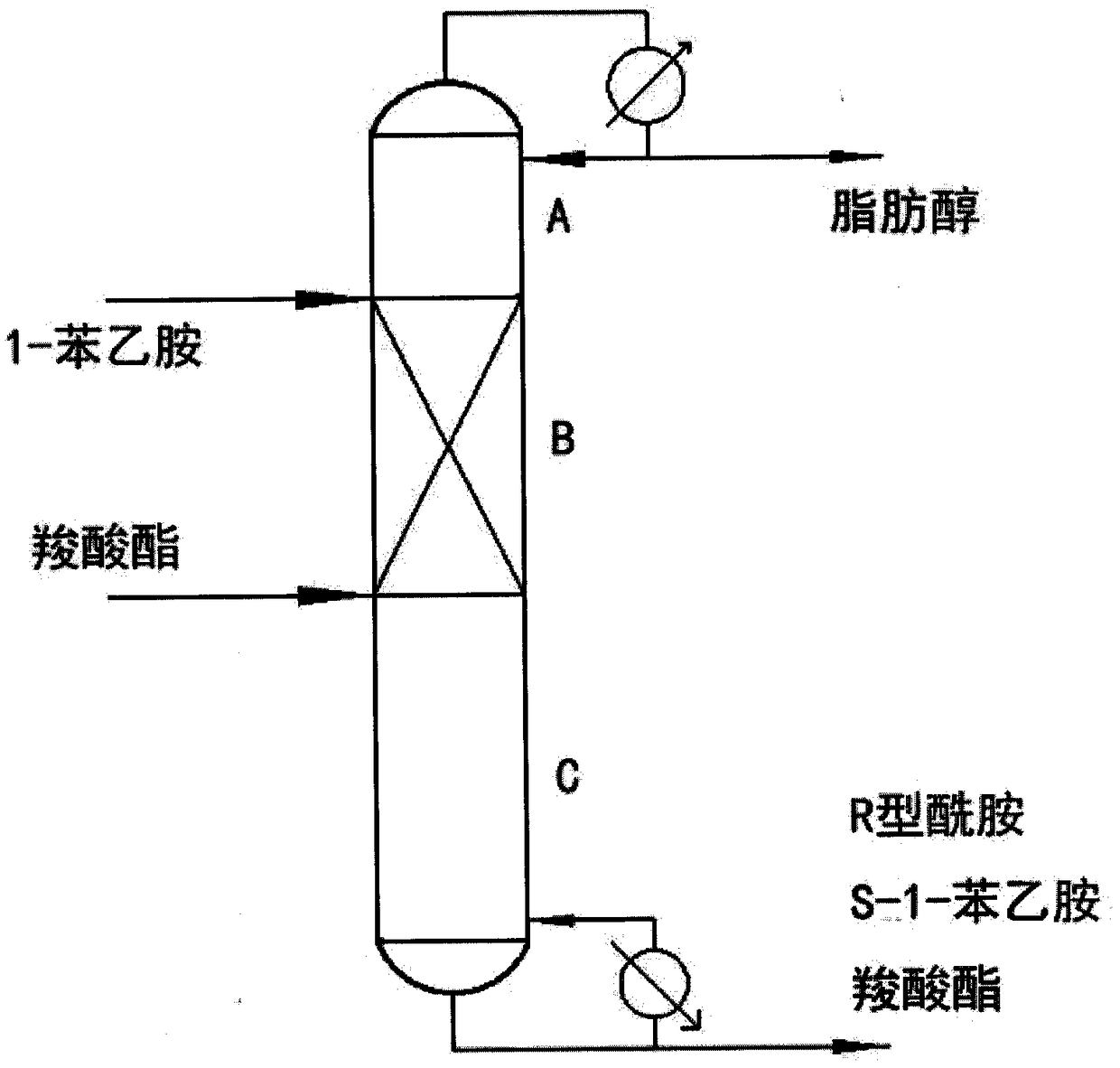
Catalytic rectification process and device for chiral resolution of 1-phenylethylamine by using lipase
InactiveCN108285419AObvious superiorityHigh reaction conversion rateAmino compound purification/separationOrganic chemistry methods1-phenylethanamineChemical reaction
The invention provides a chemical reaction separation device and method, and specifically relates to a catalytic rectification tower and process for catalyzing kinetic chiral resolution of 1-phenylethylamine by using lipase. The rectification tower comprises a condenser and a reboile; and the rectification tower is characterized in that a rectification tower reaction section is provided with a catalyst bag, and the interior of the catalyst bag is filled with the lipase supported on resin; and due to selective catalytic action of the lipase, a carboxylate ester is used as an acyl donor to reactwith R-type 1-phenylethylamine to form an R-type amide, and S-type 1-phenylethylamine does not participate in a reaction. The device and method provided by the invention can realize that a conversionrate of a reaction of the 1-phenylethylamine reaches 99% or more; the R-type amide product with enantiomeric excess (ee) of greater than 99% and the S-type 1-phenylethylamine product with enantiomeric excess (ee) of greater than 98% are obtained; and production processes are greatly simplified, and energy consumption and operating cost are reduced.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
Purifying method of (r)-n-(3,4-dimethoxy benzyl)-1-phenylethylamine l-tartrate and purifying method of (r)-n-(3,4-dimethoxy benzyl)-1-phenylethylamine
InactiveCN1821190ALow purityOrganic compounds purification/separation/stabilisation1-phenylethanaminePurification methods
The invention provided a method of industrially simply purifying (R)-N-(3,4-dimethoxybenzyl)-1-phenylethylamine whose purity is reduced by mixing decomposed products and impurities thereinto. (R)-N-(3,4-Dimethoxybenzyl)-1-phenylethylamine L-tartrate is provided. The method of purifying (R)-N-(3,4-dimethoxybenzyl)-1-phenylethylamine comprises adding L-tartaric acid to the (R)-N-(3,4-dimethoxybenzyl)-1-phenylethylamine of reduced purity to obtain (R)-N-(3,4-dimethoxybenzyl)-1-phenylethylamine L-tartrate of high purity as a crystal.
Owner:SUMITOMO CHEM CO LTD
Novel preparation of fenspiride
The invention discloses a new method for preparing fenspiride. The method is characterized in that methanol is adopted as a solvent; 1-phenylethylamine and methyl acrylate as raw material are subjected to the Michae addition and the Deckman condensation to synthesizing 1-phenyl-4-piperidone raw material with high efficiency; and 1-phenyl-4-piperidone is subjected to the Reformatsky reaction and the Curtius rearrangement to obtain the fenspiride product. The method is economic, safe and environment friendly, and is suitable for the industrial production.
Owner:宁波保税区欣诺生物技术有限公司
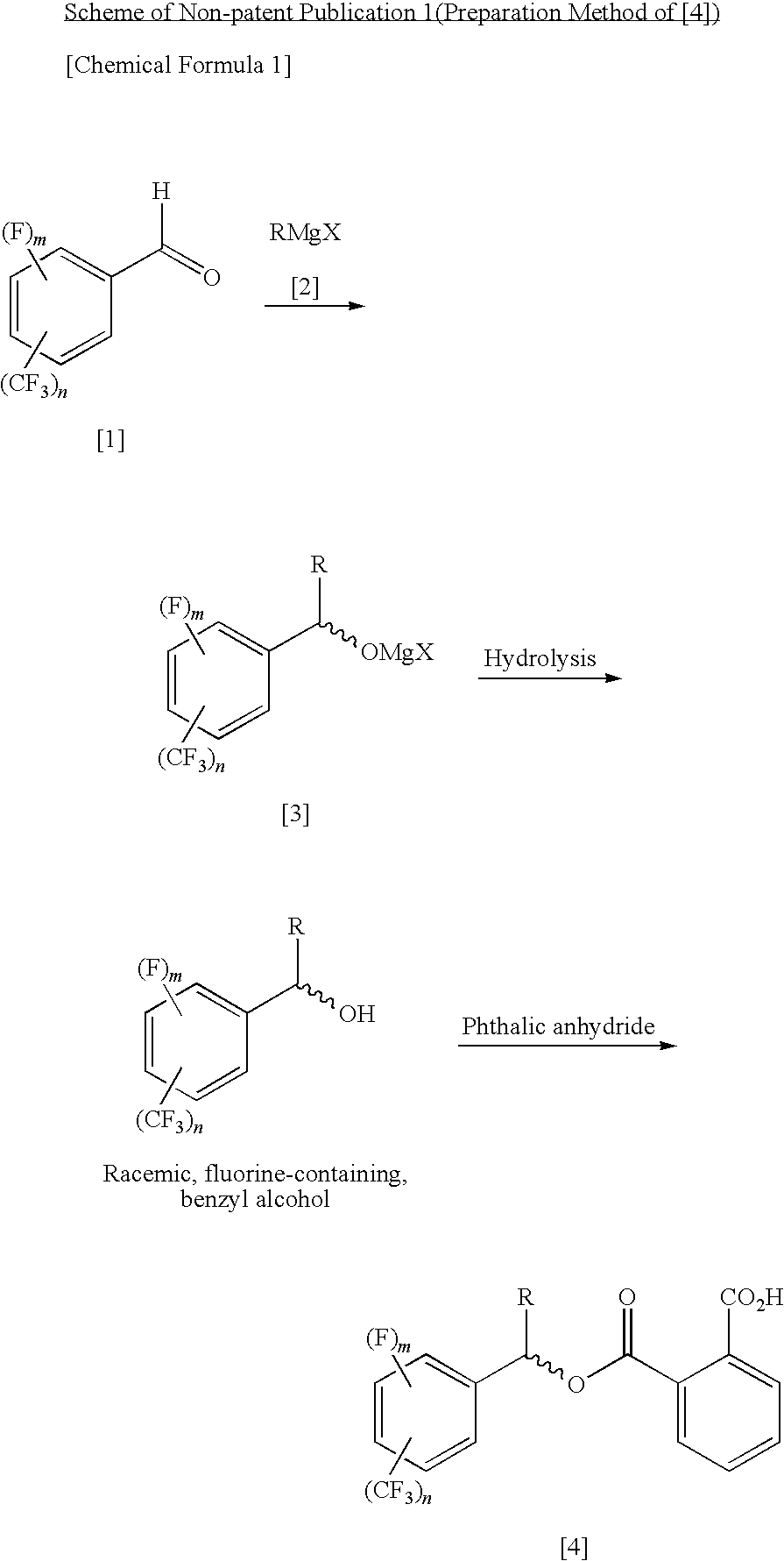
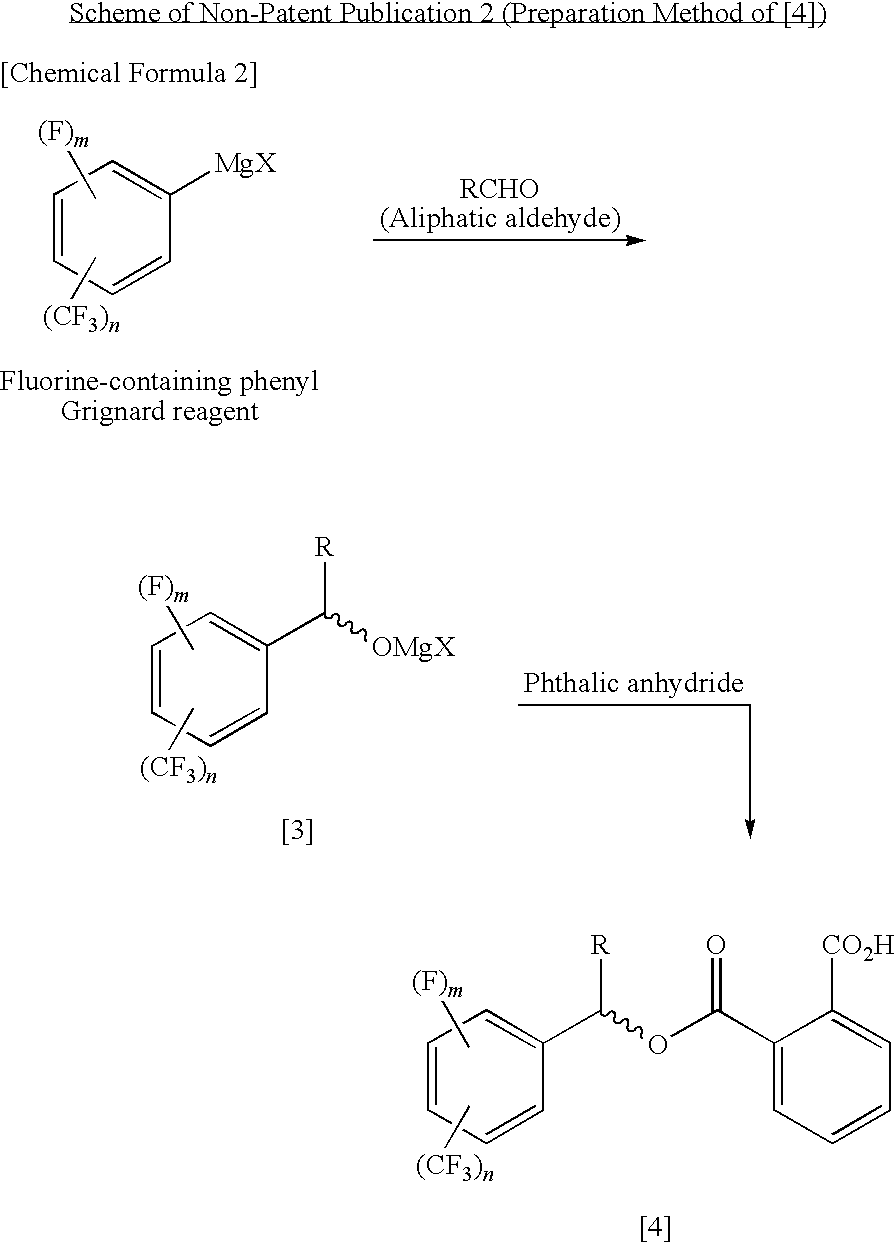
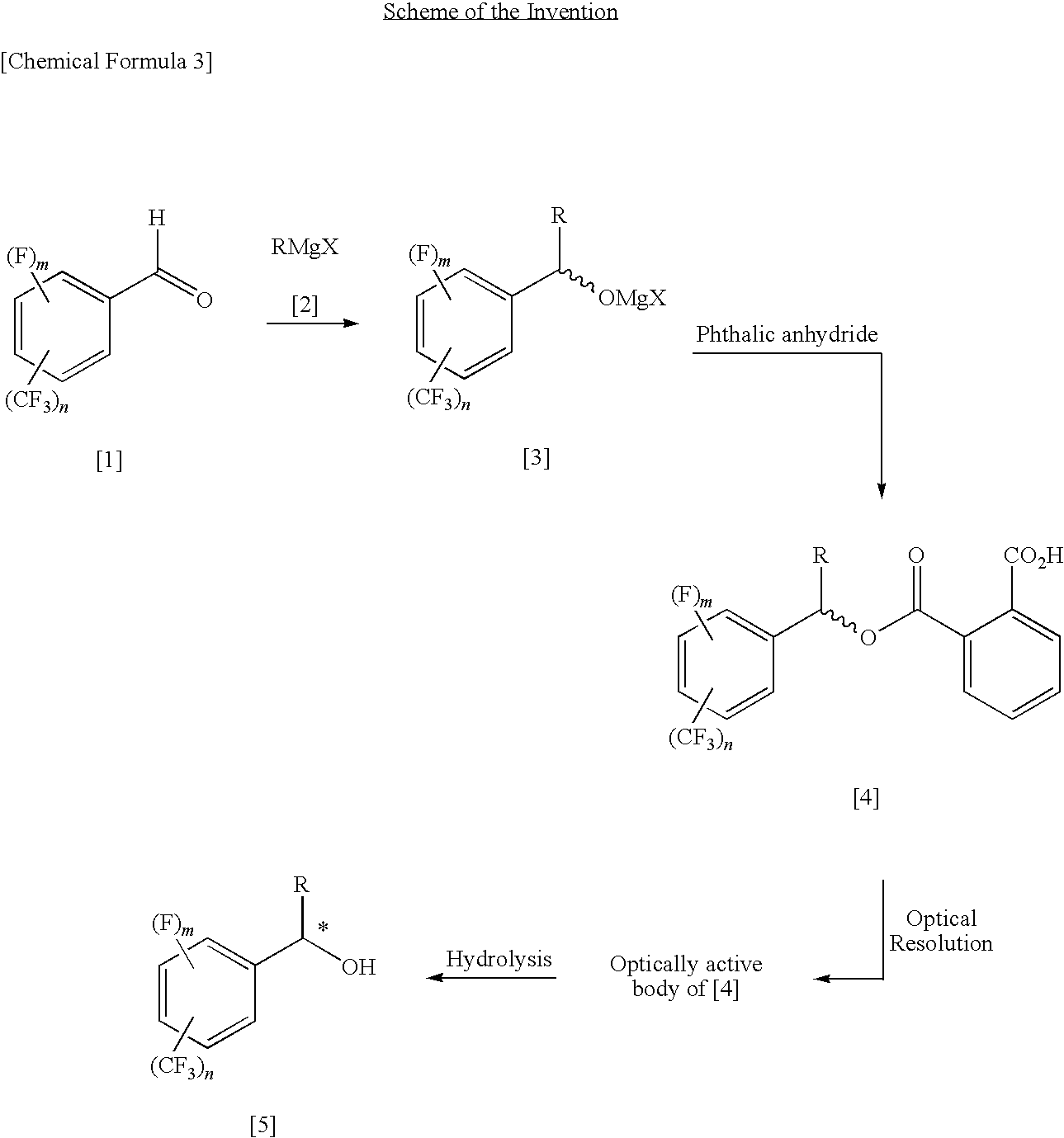
Process for Producing Optically Active Fluorobenzyl Alcohol
InactiveUS20090240087A1High yieldEasy to prepareOrganic compound preparationCarboxylic acid esters preparation1-phenylethanamineAlcohol
A fluorine-containing benzaldehyde is reacted with an alkyl Grignard reagent to convert it to a magnesium alkoxide of racemic, fluorine-containing, benzyl alcohol, and subsequently the magnesium alkoxide is reacted with phthalic anhydride to obtain a phthalate half ester of racemic, fluorine-containing, benzyl alcohol, and the half ester is optically resolved by optically active 1-phenylethylamine, and then the ester group is hydrolyzed, thereby producing an optically active, fluorine-containing, benzyl alcohol.
Owner:CENT GLASS CO LTD
Mn-Anderson type heteropolyacid catalyst modified by (S)-1-(1-phenethyl) thiourea, and preparation method and application thereof
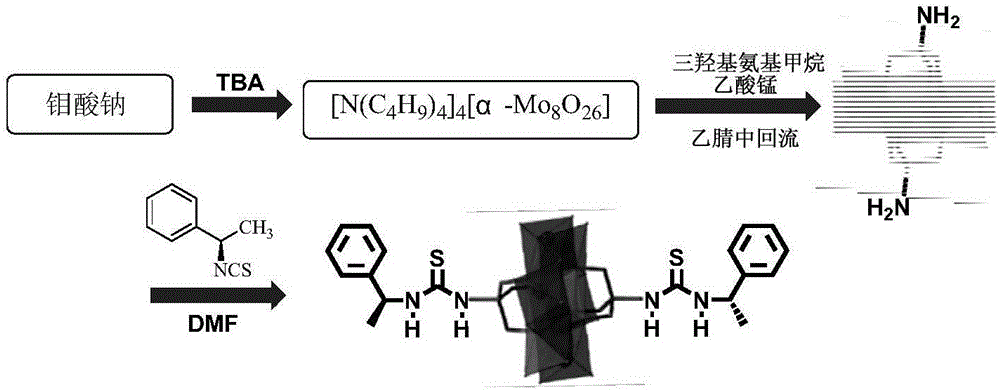
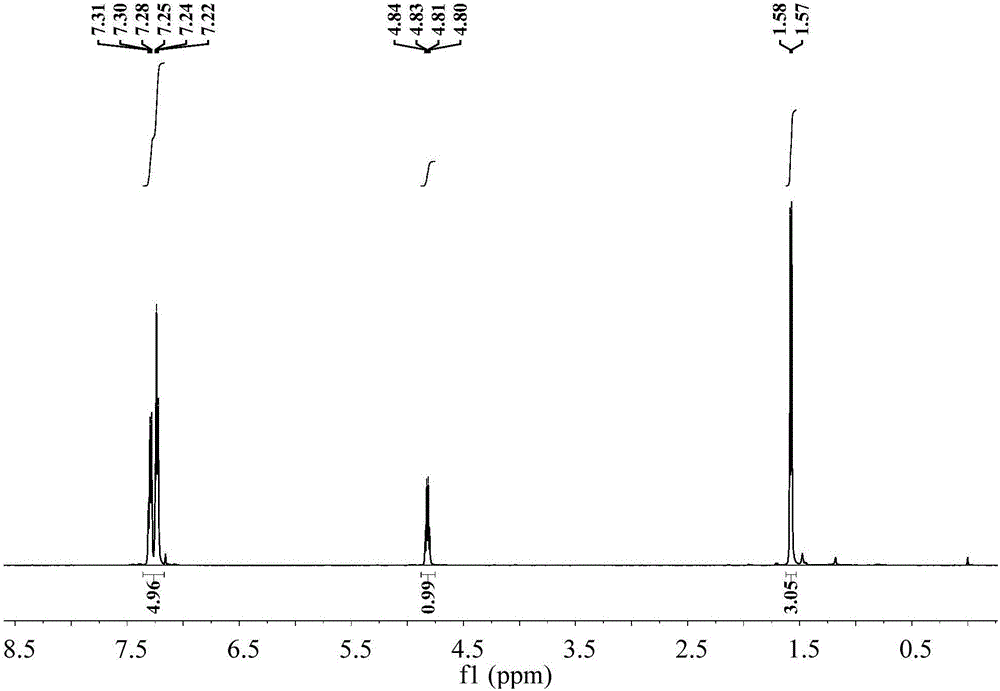
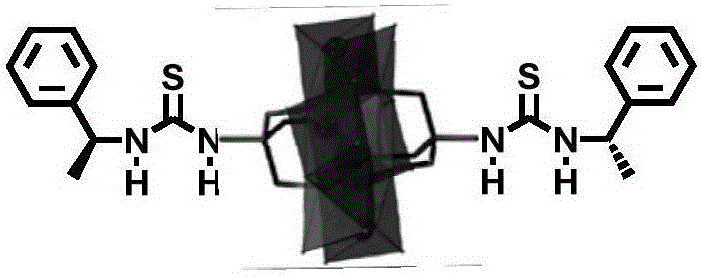
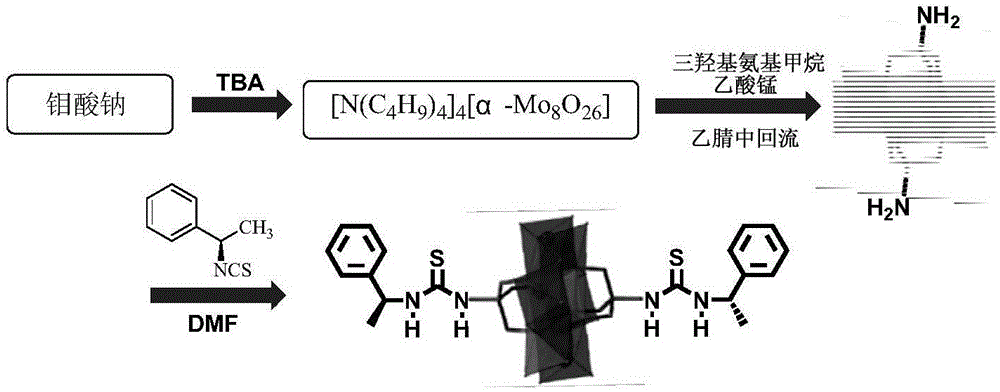
ActiveCN105772088AHydrophilicBroaden the field of studyOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsMANGANESE ACETATEThiourea
The invention discloses a Mn-Anderson type heteropolyacid catalyst modified by (S)-1-(1-phenethyl) thiourea, and a preparation method and application thereof. The preparation method comprises the following steps: firstly, reacting sodium molybdate and tetrabutylammonium bromide to generate [N(C4H9)4]4[alpha-Mo8O26], and then reacting [N(C4H9)4]4[alpha-Mo8O26] with tris(hydroxymethyl) aminomethane and manganese acetate to obtain organic bilateral amino-modified polyoxometallate; then with (R)-(+)-1-phenylethylamine as a raw material, synthesizing (S)-1-(1-phenethyl) isothiocyanate; and finally, reacting (S)-1-(1-phenethyl) isothiocyanate and organic bilateral amino-modified polyoxometallate to obtain the target catalyst. The preparation method is simple, and the reaction condition is mild and environmentally friendly; and the obtained catalyst is used for asymmetric dihydroxylation of olefin, has the advantages of high enantioselectivity, high catalytic activity, recoverability and the like and is applicable to industrial production.
Owner:上海元革新材料科技有限公司
(R)-1-(1-phenethyl) thiourea modified Mn-Anderson type heteropolyacid catalyst as well as preparation method and application thereof
ActiveCN105797771AHydrophilicBroaden the field of studyOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsMANGANESE ACETATEThiourea
The invention discloses a (R)-1-(1-phenethyl) thiourea modified Mn-Anderson type heteropolyacid catalyst as well as a preparation method and an application thereof. The preparation method comprises steps as follows: firstly, sodium molybdate and tetrabutylammonium bromide are subjected to a reaction to generate [N(C4H9)4]4[alpha-Mo8O26], [N(C4H9)4]4[alpah-Mo8O26] is subjected to a reaction with tris(hydroxymethyl)aminomethane and manganese acetate, and organic bilateral amino modified polyoxometallate is obtained; (R)-(+)-1-phenylethylamine is taken as a raw material to synthesize (R)-1-(1-phenethyl) isothiocyanic acid; finally, (R)-1-(1-phenethyl) isothiocyanic acid is subjected to a reaction with the organic bilateral amino modified polyoxometallate, and the target catalyst is obtained. The preparation method is simple, and reaction conditions are mild and environment friendliness is realized; the catalyst can be applied to asymmetric dihydroxylation of olefin, has the advantages of high enantioselectivity, high catalytic activity, recyclability and the like and is suitable for industrial production.
Owner:上海元革新材料科技有限公司
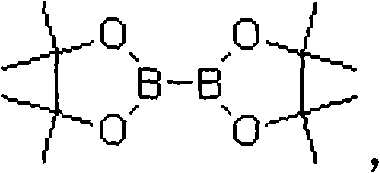
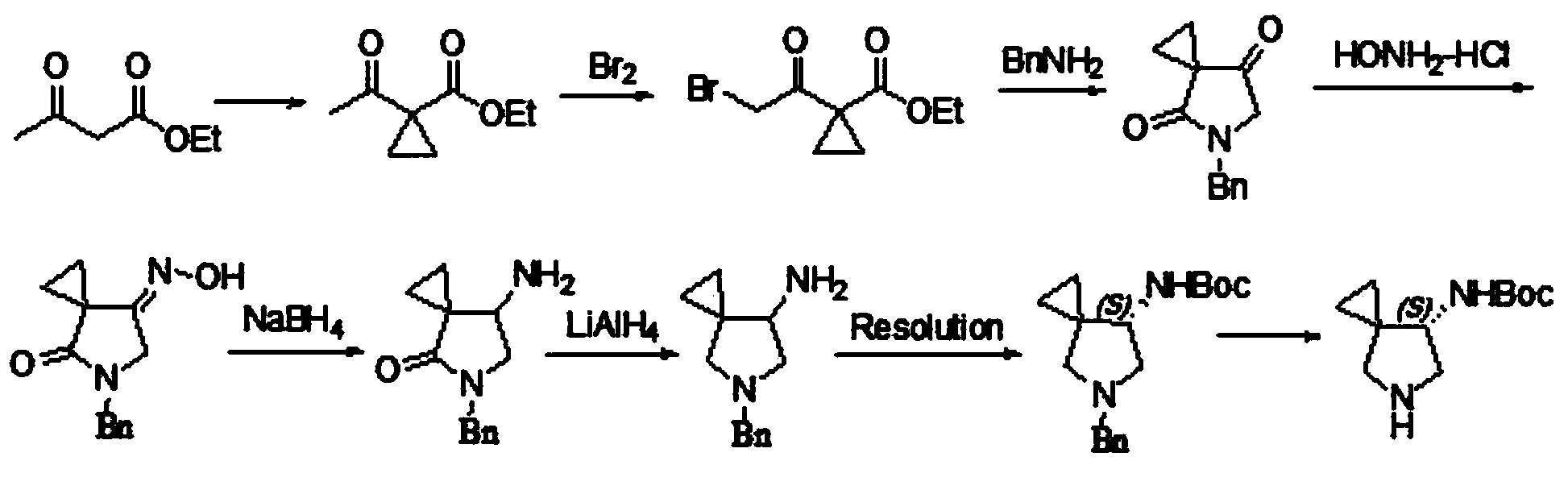
Asymmetric synthesis method, related raw material and preparation method of (s,s)-2,8-diazabicyclo[4,3,0]nonane
ActiveUS20140066626A1Reduce wasteReduce processing costsOrganic chemistryBulk chemical productionNonaneSynthesis methods
The present invention relates to an asymmetric synthesis method of a chiral intermediate (S,S)-2,8-diazabicyclo[4,3,0]nonane (I) of moxifloxacin, wherein an imide or enamine compound is obtained by dehydration reaction of the pyrrolidine-3-ketone as shown in formula (II) and chiral amine(R)-1-phenylethylamine, followed by the reduction of the imide or enamine compound to obtain a compound of formula (III) or (IV) having the chiral structure of formula (I), and then a compound of formula (I) is obtained by intramolecular cyclization, and removal of the chiral auxiliary group and amino-protecting group. The present invention also relates to pyrrolidine-3-ketone as shown in formula (II) and a preparation method therefor,and in the formula (I), (II), (III), (IV), R is an amino-protecting group, especially C1-4 alkoxycarbonyl, benzyloxycarbonyl or benzyl which can be removed by hydrolysis or hydrogenation. Z═H2 or O; when Z═H2, Y is chlorine, bromine, iodine, methanesulfonate, tosylate, hydroxyl or hydroxyl with protection; and when Z═O, Y is OR1, and R1 is C1-4 alkyl.
Owner:SHANGHAI PUYI CHEM TECH
Chiral MOC liquid chromatography separation column for resolution of racemic compounds
ActiveCN109692674AGood chiral recognition abilityEasy to separateOther chemical processesOrganic compound preparationChromatographic separationSolvent
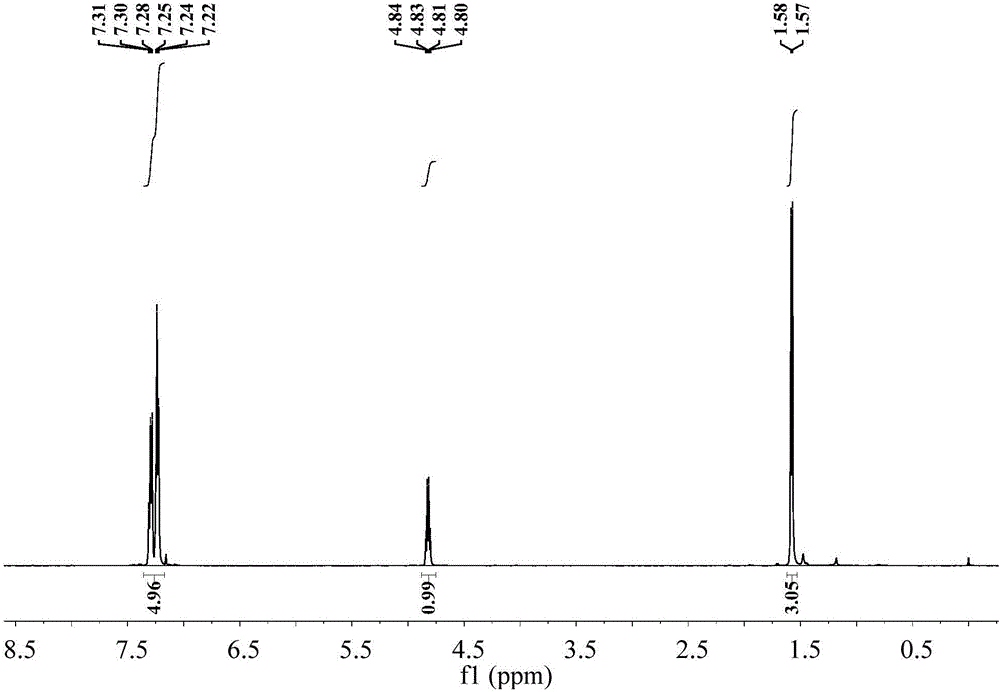
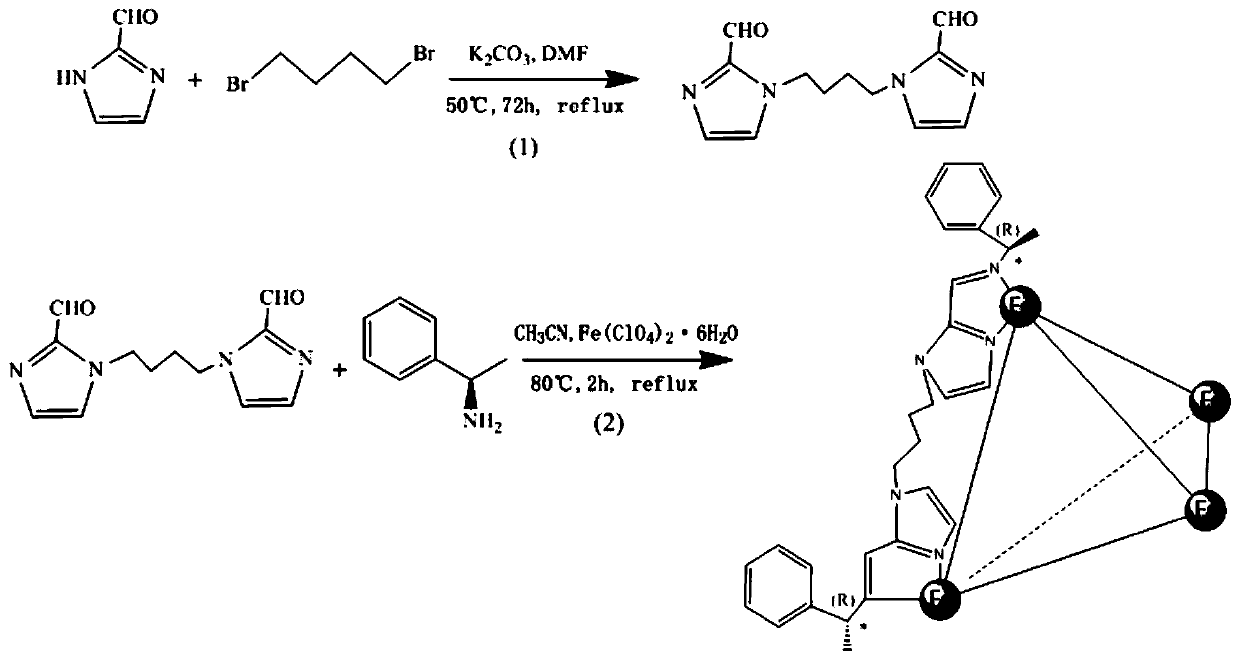
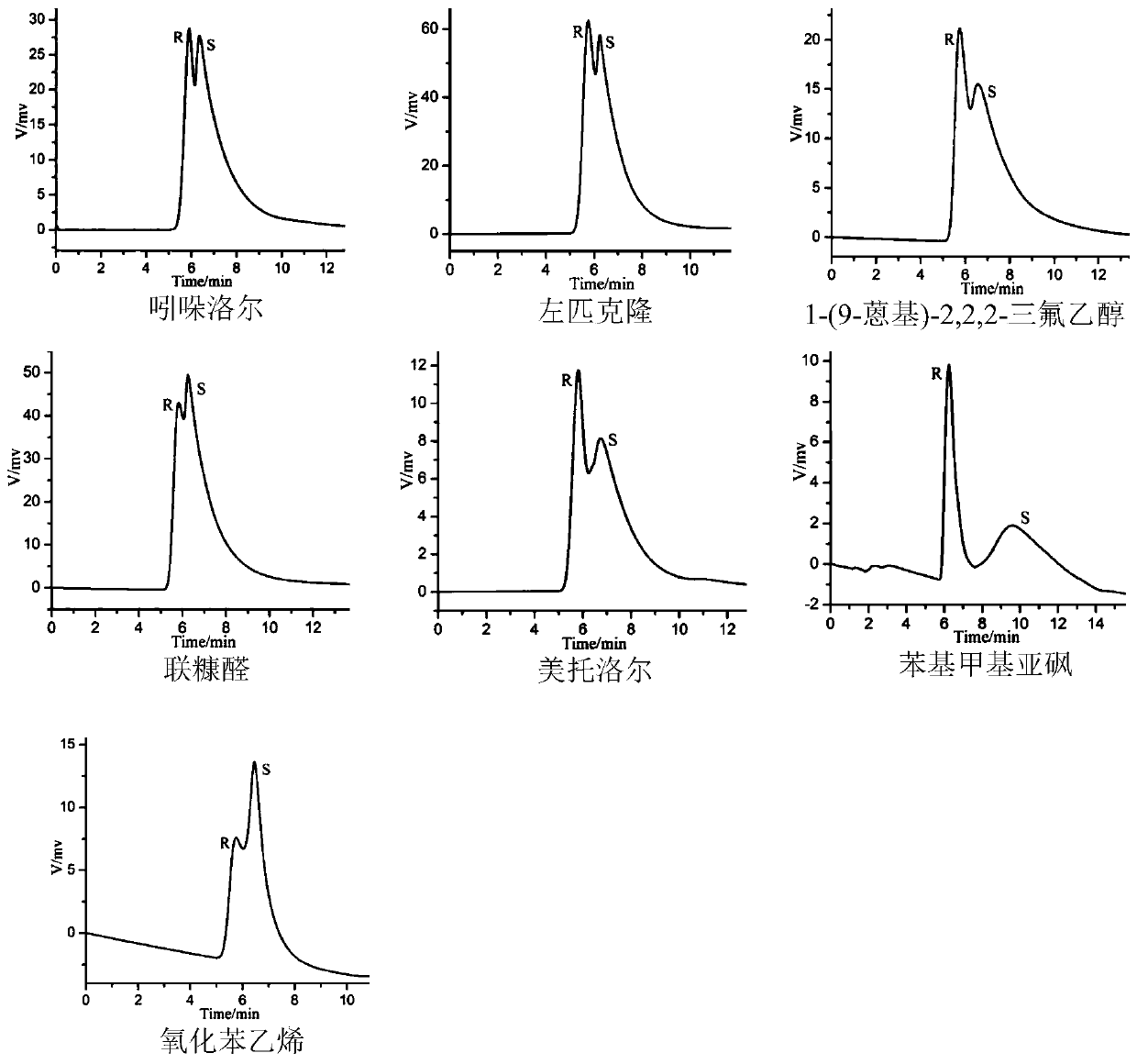
The invention discloses a chiral MOC liquid chromatography separation column for the resolution of racemic compounds. Chiral metal organic cage [Fe4L6](ClO4)8.Solvent particles are used as the stationary phase of high performance liquid chromatography, and chiral metal organic cage [Fe4L6](ClO4)8.Solvent crystals are formed by molecularly self-assembling 1,4-di(2- imidazolecarboxaldehyde)butane, (R)-1-phenylethylamine and Fe(ClO4)2.6H2O. The chiral column has good chiral recognition ability for seven racemic compounds. The chiral metal organic cages (MOCs) used as the stationary phase for liquid chromatography separation to resolute the racemic compounds have the advantages of cheap and easily available materials for synthesis, low column production cost, high separation speed, good stability and repeated use, open up a new way for the application of porous materials in the field of separation science, and have a good application prospect in the field of chiral recognition.
Owner:YUNNAN NORMAL UNIV
(r)-1-(1-phenylethyl)thiourea-modified mn-anderson type heteropolyacid catalyst, preparation method and application thereof
ActiveCN105797771BHigh enantioselectivityHigh stereoselectivityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsMANGANESE ACETATEThiourea
The invention discloses a (R)-1-(1-phenethyl) thiourea modified Mn-Anderson type heteropolyacid catalyst as well as a preparation method and an application thereof. The preparation method comprises steps as follows: firstly, sodium molybdate and tetrabutylammonium bromide are subjected to a reaction to generate [N(C4H9)4]4[alpha-Mo8O26], [N(C4H9)4]4[alpah-Mo8O26] is subjected to a reaction with tris(hydroxymethyl)aminomethane and manganese acetate, and organic bilateral amino modified polyoxometallate is obtained; (R)-(+)-1-phenylethylamine is taken as a raw material to synthesize (R)-1-(1-phenethyl) isothiocyanic acid; finally, (R)-1-(1-phenethyl) isothiocyanic acid is subjected to a reaction with the organic bilateral amino modified polyoxometallate, and the target catalyst is obtained. The preparation method is simple, and reaction conditions are mild and environment friendliness is realized; the catalyst can be applied to asymmetric dihydroxylation of olefin, has the advantages of high enantioselectivity, high catalytic activity, recyclability and the like and is suitable for industrial production.
Owner:上海元革新材料科技有限公司
Method for split preparation of S-3,4-(methylenedioxy) mandelic acid
The invention discloses a method for split preparation of S-3,4-(methylenedioxy) mandelic acid. The method comprises the steps that racemic 3,4-(methylenedioxy) mandelic acid and resolving agents of S-1-phenylethylamine take a reaction to obtain S-1-phenylethylamine salts of S-3,4-(methylenedioxy) mandelic acid; the salts are subjected to acidification, organic solvent extraction and concentration to obtain S-3,4-(methylenedioxy) mandelic acid. All solutions containing S-1-phenylethylamine ingredients are subjected to alcohol removal after being mixed, and the processed solutions are subjected to alkalization, organic solvent extraction and concentration to recover the S-1-phenylethylamine. The method has the characteristics that the conditions are mild; the operation is simple; the obtained product has high yield and high optical purity; the resolving agents can be recovered and cyclically utilized, and the like. The method is particularly suitable for being used for preparing and producing the S-3,4-(methylenedioxy) mandelic acid.
Owner:彭静
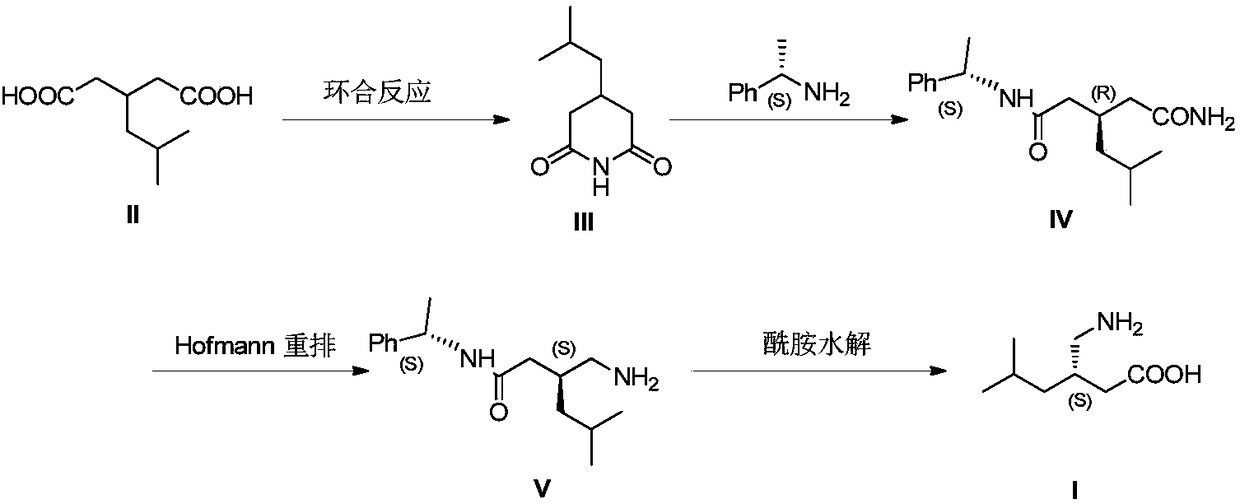
Asymmetrical synthesis method of lyrica
InactiveCN108069866AFew reaction stepsHigh yieldOrganic compound preparationCarboxylic acid amides preparation1-phenylethanamineSynthesis methods
The invention discloses an asymmetrical synthesis method of lyrica, wherein the synthesis steps comprise: carrying out a cyclic anhydridization reaction by using 3-isobutylglutaric acid as a raw material, carrying out an asymmetric ring-opening reaction with (R)-(+)-1-phenylethylamine, and sequentially carrying out a hydrogenation reaction and Huffman rearrangement to obtain lyrica. Compared to the synthesis method in the prior art, the synthesis method of the present invention has advantages of inexpensive and easily-available raw materials and less reaction steps, has the total yield of up to 60%, the purity of the product lyrica of more than 99% and the ee value of more than 99%, and has good application prospect in industrial scale up production.
Owner:SYNCOZYMES SHANGHAI
Asymmetric synthesis method, related raw material and preparation method of (S,S)-2,8-diazabicyclo[4,3,0]nonane
ActiveUS9238648B2Reduce wasteReduce processing costsOrganic chemistryBulk chemical productionNonaneSynthesis methods
Owner:SHANGHAI PUYI CHEM TECH
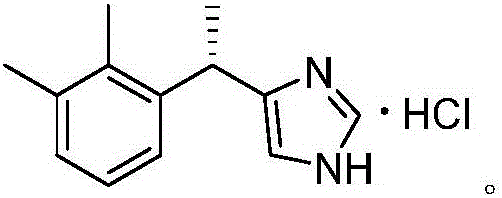
Preparation method of dexmedetomidine hydrochloride for sedation and analgesia in ICU
InactiveCN106749030AOvercome the defect of low split efficiencyHigh yieldOrganic chemistryBenzene1-phenylethanamine
The invention discloses a preparation method of dexmedetomidine hydrochloride for sedation and analgesia in an ICU. The preparation method comprises the following steps: (1) reacting by virtue of racemic 4-[1-(2,3-dimethylphenyl)ethyl]-1H-imidazole and liquid sulfur trioxide in 1,4-dioxane, so as to obtain sulfonated 4-[1-(2,3-dimethylphenyl)ethyl]-1H-imidazole; (2) reacting by virtue of sulfonated 4-[1-(2,3-dimethylphenyl)ethyl]-1H-imidazole and (S)-1-phenylethylamine or benzene ring substituted (S)-1-phenylethylamine, so as to obtain (S)-sulfonated-4-[1-(2,3-dimethylphenyl)ethyl]-1H-aminoimidazole; and (3) carrying out dichloromethane extraction in a sodium hydroxide solution, concentrating, and stirring in a hydrochloric acid solution, so as to obtain dexmedetomidine hydrochloride. The preparation method has the beneficial effects that sulfonation is carried out firstly, high-yield chiral phenylethylamine resolution is then carried out to obtain an (S)-product, conditions in each step are mild, and the preparation method is simple, feasible and suitable for industrial production.
Owner:禚修洁 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

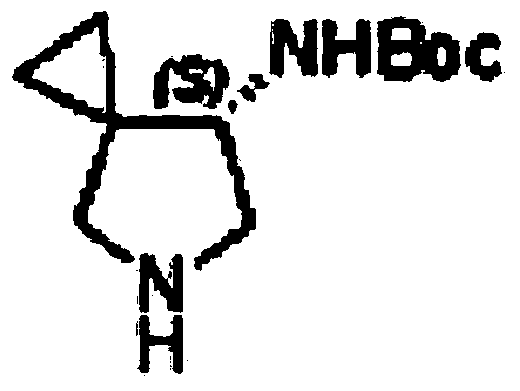
![Asymmetric synthesis method, related raw material and preparation method of (s,s)-2,8-diazabicyclo[4,3,0]nonane Asymmetric synthesis method, related raw material and preparation method of (s,s)-2,8-diazabicyclo[4,3,0]nonane](https://images-eureka.patsnap.com/patent_img/c7dc59e8-6dfe-4ef6-9482-e7a7fe659381/US20140066626A1-20140306-C00001.png)
![Asymmetric synthesis method, related raw material and preparation method of (s,s)-2,8-diazabicyclo[4,3,0]nonane Asymmetric synthesis method, related raw material and preparation method of (s,s)-2,8-diazabicyclo[4,3,0]nonane](https://images-eureka.patsnap.com/patent_img/c7dc59e8-6dfe-4ef6-9482-e7a7fe659381/US20140066626A1-20140306-C00002.png)
![Asymmetric synthesis method, related raw material and preparation method of (s,s)-2,8-diazabicyclo[4,3,0]nonane Asymmetric synthesis method, related raw material and preparation method of (s,s)-2,8-diazabicyclo[4,3,0]nonane](https://images-eureka.patsnap.com/patent_img/c7dc59e8-6dfe-4ef6-9482-e7a7fe659381/US20140066626A1-20140306-C00003.png)
![Asymmetric synthesis method, related raw material and preparation method of (S,S)-2,8-diazabicyclo[4,3,0]nonane Asymmetric synthesis method, related raw material and preparation method of (S,S)-2,8-diazabicyclo[4,3,0]nonane](https://images-eureka.patsnap.com/patent_img/4cfdc7a0-0777-4ba6-af1a-98321dba831d/US09238648-20160119-C00001.PNG)
![Asymmetric synthesis method, related raw material and preparation method of (S,S)-2,8-diazabicyclo[4,3,0]nonane Asymmetric synthesis method, related raw material and preparation method of (S,S)-2,8-diazabicyclo[4,3,0]nonane](https://images-eureka.patsnap.com/patent_img/4cfdc7a0-0777-4ba6-af1a-98321dba831d/US09238648-20160119-C00002.PNG)
![Asymmetric synthesis method, related raw material and preparation method of (S,S)-2,8-diazabicyclo[4,3,0]nonane Asymmetric synthesis method, related raw material and preparation method of (S,S)-2,8-diazabicyclo[4,3,0]nonane](https://images-eureka.patsnap.com/patent_img/4cfdc7a0-0777-4ba6-af1a-98321dba831d/US09238648-20160119-C00003.PNG)