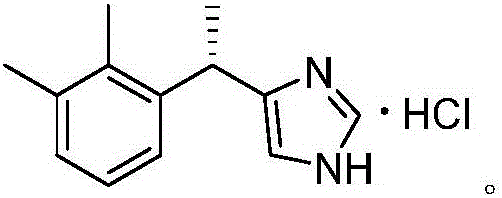
Preparation method of dexmedetomidine hydrochloride for sedation and analgesia in ICU
A technology for dexmedetomidine hydrochloride and sedation, which is applied in the field of preparation of dexmedetomidine hydrochloride, can solve the problems of unsatisfactory splitting yield of a racemate intermediate, and achieves easy industrial production, high yield, high yield, and the like. Step condition mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
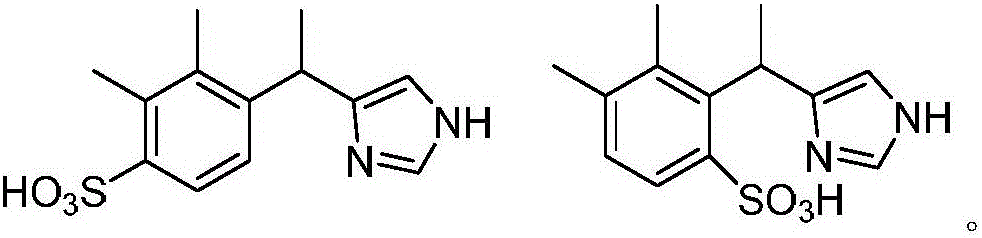
[0028] Preparation of sulfonated 4-[1-(2,3-dimethylphenyl)ethyl]-1H-imidazole;
[0029] Add 20 g (100 mmol) of racemic 4-[1-(2,3-dimethylphenyl) ethyl]-1H-imidazole and 3 g of sodium sulfate into 100 ml of 1,4-dioxane, and then slowly Raise the temperature to 90°C, add 5.1ml (120mmol) of sulfur trioxide dropwise under stirring, continue the reaction for 1-2 hours, monitor the end of the reaction, concentrate the reaction solution under reduced pressure, wash the concentrate with cold ethanol (0-5°C) 27.6 g of sulfonated 4-[1-(2,3-dimethylphenyl)ethyl]-1H-imidazole was obtained with a yield of 98.4%.
Embodiment 2
[0031] Preparation of sulfonated 4-[1-(2,3-dimethylphenyl)ethyl]-1H-imidazole;
[0032] Add 20 g (100 mmol) of racemic 4-[1-(2,3-dimethylphenyl) ethyl]-1H-imidazole and 3 g of sodium sulfate into 100 ml of 1,4-dioxane, and then slowly Raise the temperature to 90°C, add 5.5ml (130mmol) of sulfur trioxide dropwise under stirring, continue the reaction for 1-2 hours, monitor the end of the reaction, concentrate the reaction solution under reduced pressure, wash the concentrate with cold ethanol (0-5°C) 27.8 g of sulfonated 4-[1-(2,3-dimethylphenyl)ethyl]-1H-imidazole was obtained with a yield of 99.1%.
Embodiment 3
[0034] Preparation of sulfonated 4-[1-(2,3-dimethylphenyl)ethyl]-1H-imidazole;
[0035] Add 200 g (1 mol) of racemic 4-[1-(2,3-dimethylphenyl) ethyl]-1H-imidazole and 2 g of sodium sulfate into 950 ml of 1,4-dioxane, then slowly Slowly raise the temperature to 80°C, add 63ml (1.5mol) of sulfur trioxide dropwise under stirring, continue the reaction for 1-2 hours, monitor the end of the reaction, concentrate the reaction solution under reduced pressure, and use cold ethanol (0-5°C) for the concentrate After washing, 276.7 g of sulfonated 4-[1-(2,3-dimethylphenyl)ethyl]-1H-imidazole was obtained, with a yield of 98.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com