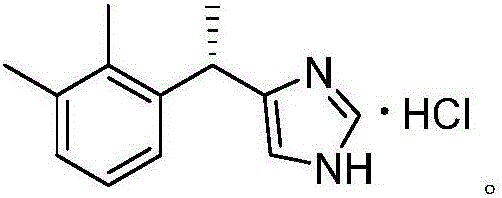
Dexmedetomidine hydrochloride intermediate resolution method
A technology for dexmedetomidine hydrochloride and intermediates, which is applied in the field of enantiomeric separation and detection, can solve problems such as unsatisfactory direct separation of medetomidine, and achieve favorable raw material supply guarantee, huge market potential, and high efficiency. Yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Preparation of sulfonated 4-[1-(2,3-dimethylphenyl)ethyl]-1H-imidazole;
[0024] In a 250ml three-necked flask, add 40g (200mmol) racemic 4-[1-(2,3-dimethylphenyl)ethyl]-1H-imidazole, 4g sodium sulfate and 120ml 1,4-dioxane Ring, then slowly be warming up to 90 ℃, add sulfur trioxide 10.2ml (240mmol) dropwise under stirring, dripping is finished, continue to react for 2 hours, monitor the reaction to finish, concentrate the reaction solution under reduced pressure, the concentrate uses cold ethanol (0~5 °C) washing to obtain 55.4 g of sulfonated 4-[1-(2,3-dimethylphenyl)ethyl]-1H-imidazole with a yield of 98.7%.
Embodiment 2
[0026] Preparation of sulfonated 4-[1-(2,3-dimethylphenyl)ethyl]-1H-imidazole;
[0027] In a 250ml three-necked flask, add 20g (100mmol) racemic 4-[1-(2,3-dimethylphenyl)ethyl]-1H-imidazole, 3g sodium sulfate and 80ml 1,4-dioxane Ring, then slowly be warming up to 80 ℃, add sulfur trioxide 6.4ml (150mmol) dropwise under stirring, dripping is completed, continue to react for 2 hours, monitor the reaction to finish, concentrate the reaction solution under reduced pressure, and the concentrate uses cold ethanol (0~5 °C) washing to obtain 27.7 g of sulfonated 4-[1-(2,3-dimethylphenyl)ethyl]-1H-imidazole with a yield of 98.9%.
Embodiment 3
[0029] Preparation of sulfonated 4-[1-(2,3-dimethylphenyl)ethyl]-1H-imidazole;
[0030] In a 250ml three-necked flask, add 20g (100mmol) racemic 4-[1-(2,3-dimethylphenyl)ethyl]-1H-imidazole, 2g sodium sulfate and 80ml 1,4-dioxane Ring, then slowly heat up to 60 ° C, add 5.1 ml (120 mmol) of sulfur trioxide dropwise under stirring, and continue to react for 6 hours after dropping, and concentrate the reaction solution under reduced pressure, and the concentrate is washed with cold ethanol (0 ~ 5 ° C) to obtain Sulfonated 4-[1-(2,3-dimethylphenyl)ethyl]-1H-imidazole 25.1 g, yield 89.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com