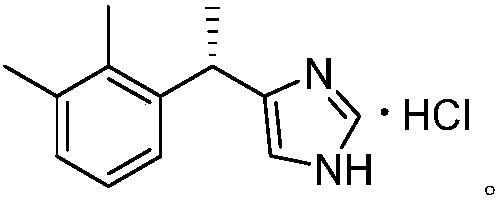
A kind of resolution method of dexmedetomidine hydrochloride intermediate
A technology for dexmedetomidine hydrochloride and intermediates, which is applied in the field of enantiomer resolution and detection, can solve problems such as unsatisfactory direct resolution of medetomidine, and achieve favorable raw material supply guarantee and high resolution yield , the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Preparation of sulfonated 4-[1-(2,3-dimethylphenyl)ethyl]-1H-imidazole;
[0024] In a 250ml three-necked flask, add 40g (200mmol) racemic 4-[1-(2,3-dimethylphenyl)ethyl]-1H-imidazole, 4g sodium sulfate and 120ml 1,4-dioxane Then slowly increase the temperature to 90°C, add 10.2ml (240mmol) of sulfur trioxide dropwise under stirring, and continue the reaction for 2 hours. Monitor the end of the reaction. Concentrate the reaction solution under reduced pressure. Use cold ethanol (0~5 (°C) washing to obtain 55.4 g of sulfonated 4-[1-(2,3-dimethylphenyl)ethyl]-1H-imidazole with a yield of 98.7%.
Embodiment 2
[0026] Preparation of sulfonated 4-[1-(2,3-dimethylphenyl)ethyl]-1H-imidazole;
[0027] In a 250ml three-necked flask, add 20g (100mmol) racemic 4-[1-(2,3-dimethylphenyl)ethyl]-1H-imidazole, 3g sodium sulfate and 80ml 1,4-dioxane Then slowly increase the temperature to 80°C, add 6.4ml (150mmol) of sulfur trioxide dropwise with stirring, continue the reaction for 2 hours, monitor the end of the reaction, concentrate the reaction solution under reduced pressure, and use cold ethanol (0~5 (°C) washing to obtain 27.7 g of sulfonated 4-[1-(2,3-dimethylphenyl)ethyl]-1H-imidazole with a yield of 98.9%.
Embodiment 3
[0029] Preparation of sulfonated 4-[1-(2,3-dimethylphenyl)ethyl]-1H-imidazole;
[0030] In a 250ml three-necked flask, add 20g (100mmol) racemic 4-[1-(2,3-dimethylphenyl)ethyl]-1H-imidazole, 2g sodium sulfate and 80ml 1,4-dioxane Then slowly warm up to 60°C, add 5.1ml (120mmol) of sulfur trioxide dropwise with stirring, continue the reaction for 6 hours, concentrate the reaction solution under reduced pressure, and wash the concentrate with cold ethanol (0~5°C). Sulfonated 4-[1-(2,3-dimethylphenyl)ethyl]-1H-imidazole was 25.1 g, and the yield was 89.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com