Improved method used for preparing ambrisentan
A technology of ambrisentan and metal carbonate, which is applied in the field of chemistry, can solve the problems of cumbersome operation, unsuitability for industrial production, and expensive resolution reagents, and achieve simplified reaction and processing steps, cheap and easy-to-obtain resolution reagents, and The effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Preparation of methyl 3,3-diphenyl-2,3-epoxypropionate
[0032] Chemical reaction formula:
[0033]
[0034] Add tetrahydrofuran (16 L), benzophenone (4 kg) and sodium methoxide (2.96 kg) into the reaction kettle, stir well, continue to add methyl chloroacetate (5.96 kg), after the reaction is completed, add methyl Methyl tert-butyl ether (MTBE) (8 L) and water (10 L), the organic layer was washed with saturated brine and dried with a desiccant, and the organic solvent was concentrated to give the oil 3,3-diphenyl-2,3- Methyl glycidate (5.54 kg).
[0035] Mass Spectrum (ESI): m / z 255.1 (M+H) + .
[0036] 1 H NMR (400 MHz, CDCl 3 ) δ: 7.48~7.45 (m, 2H), 7.40~7.32 (m, 8H), 4.02 (s, 1H), 3.54 (s, 3H).
Embodiment 2
[0038] Preparation of methyl 2-hydroxy-3-methoxy-3,3-diphenylpropionate
[0039] chemical reaction formula
[0040]
[0041] The oily product 3,3-diphenyl-2,3-epoxypropionate methyl ester (5.54kg) and methanol (15 L) in Example 1 were put into the reaction kettle, and boron trifluoride ether was added dropwise (BF 3 -ether) (148 ml), after the reaction was completed, water (15L) was added to the reaction system to quench the reaction, the reaction solution was extracted three times with ether (7L) and the organic layer was combined, the organic layer was cooled to 0°C, filtered, and the solid was collected. Dry to obtain 5.33 kg of methyl 2-hydroxy-3-methoxy-3,3-diphenylpropionate, and the two-step yield is 85%.
[0042] Mass spectrum (ESI): m / z 255.1 (M-MeO) + .
[0043] 1 H NMR (400 MHz, DMSO- d 6) δ: 7.38 ~7.35 (m, 4H), 7.31 ~ 7.22 (m, 6H), 5.80 (d, J = 4 Hz, 1H), 5.24 (d, J = 4 Hz, 1H), 3.41 (s, 3H), 3.22 (s, 3H).
Embodiment 3
[0045] Preparation of Ambrisentan Racemate
[0046] Chemical reaction formula:
[0047]
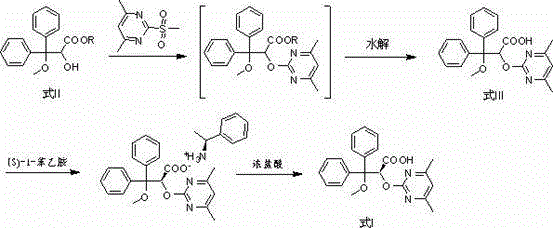
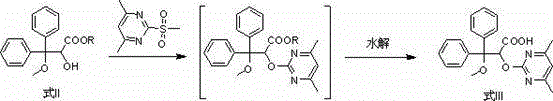
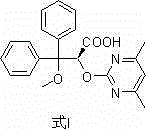
[0048] Add tetrahydrofuran (19.4 L), 3,3-diphenyl-2,3-epoxypropionic acid methyl ester (4.64 kg), 4,6-dimethyl-2-methylsulfonyl pyrimidine (3.32 kg) and potassium carbonate (3.36 kg), stir evenly, and heat to reflux. After completion of the reaction, add water (13.7 kg) to the reaction system, stir and separate the liquid, and remove the water layer; then add 2.5mol / L potassium hydroxide solution (18.1L) to the reaction system, and heat to reflux. Remove tetrahydrofuran, then add water (29 L) and dichloromethane (11.7 L), stir and separate, remove the dichloromethane layer, neutralize the water layer with hydrochloric acid to pH 1~5, filter, collect the solid, and dry to obtain Ambrisentan racemate (5.9 kg), the yield was 96%, and the purity was 99%.
[0049] Mass Spectrum (ESI): m / z 377.0 (M-H) - .
[0050] 1 H NMR (400 MHz, DMSO- d 6 ) δ: 12.56 (s, 1H), 7.35 ~ 7.19 (m, 10H)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com