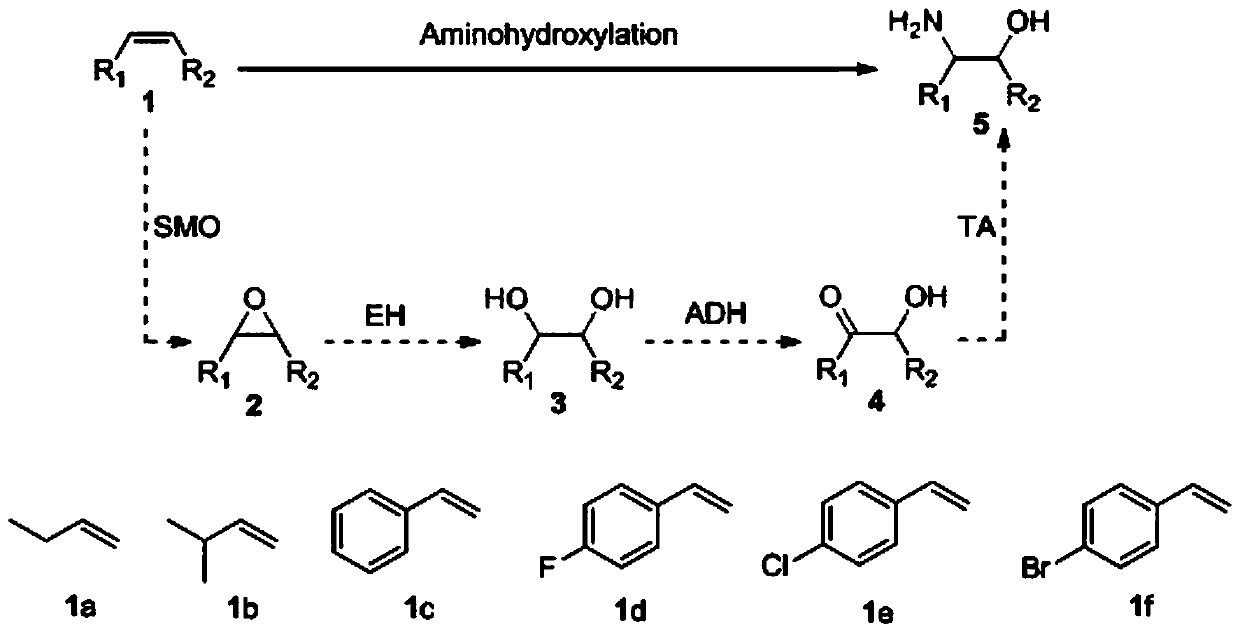
Method for preparing chiral beta-amino alcohol by asymmetric amine hydrogxylation of olefin through cascade biocatalysis
A technology for biocatalyzing alkenes and aminoalcohols, applied in the biological field, can solve problems such as difficult to find with good activity and enantioselectivity, cofactor regeneration, etc., and achieve the effects of avoiding losses, saving costs, and high catalytic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
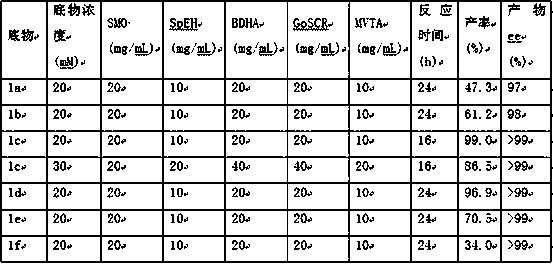
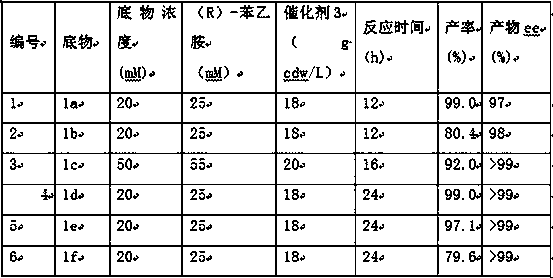
Examples
experiment example 1
[0045] Experimental Example 1: Construction of a recombinant expression vector:
[0046] SpEH gene upstream primer is CGC GGATCC GATGAAGTCGAACATATCCG, the downstream primer is CCC AAGCTT TCAAAGATCCATCTGTGCAAAGGC; primers were synthesized by Sangon Bioengineering (Shanghai) Co., Ltd., the underlined part is Bam H I and Hind III restriction site, and then use the plasmid pET28a-SpEH as a template to amplify the SpEH gene by PCR. The PCR amplification system was: 40 µL of sterilized distilled water, 5 µL of 10×Taq plus buffer, 1 µL of dNTPs, 1 µL of template, 1 µL of upstream primer, 1 µL of downstream primer, and 1 µL of Taq plus DNA polymerase. The PCR conditions are as follows: preheat the PCR instrument at 95° C. for 5 minutes to fully denature the template DNA, and then enter the amplification cycle. In each cycle, first keep at 94°C for 45 seconds to denature the template, then lower the temperature to the annealing temperature of 65°C and hold for 40 seconds to ful...
experiment example 2
[0048] Experimental example 2: Construction of co-expression vector
[0049] Ligate the gene SpEH into another polyclonal region of the recombinant plasmid pRSFDuet-SMO using restriction enzymes Ned I and xho I performed double enzyme digestion on the gene SpEH and the recombinant plasmid pRSFDuet-SMO, and the enzyme digestion conditions were the same as in Experimental Example 1. then use T 4 DNA ligase was used to connect the target gene SpEH and the expression vector pRSFDuet-SMO at 4 °C to construct the recombinant plasmid pRSFDuet-SMO-SpEH, which was introduced into E. coli DH5 by heat shock method α Competent cells were spread on LB solid medium plates containing kanamycin (50 μg / mL) and cultured overnight at 37 °C. After the colonies grow, randomly pick the single-clonal transformant on the resistant plate and put it in the LB liquid medium containing the corresponding antibiotic, culture it on a shaker at 37 ℃ for 12-16 h, extract the plasmid, and perform plasmid...
experiment example 3
[0051] Experimental example 3: Expression of Escherichia coli recombinant bacteria
[0052] For the expression of Escherichia coli recombinant bacteria, the following method is used in the present invention: inoculate the strain constructed in Experimental Example 1 into LB medium containing corresponding antibiotics (kanamycin or ampicillin), at 37°C, 200 rpm Shake culture for 8 hours, transfer 2% inoculum into a shake flask containing 45 mL of TB medium, add 5 mL of sterilized phosphate buffer; culture at 37 °C, 200 rpm, when the OD of the culture solution 600 When reaching 0.6, add IPTG with a final concentration of 0.1 mM, and induce for 12 h at 20 °C and 200 rpm. The cells were then collected by centrifugation at 8000 rpm for 5 min at 4 °C, and washed twice with 100 mM sodium phosphate buffer, pH 7.0. Suspend the obtained bacteria with 100 mM sodium phosphate buffer solution with a pH of 7.5, sonicate them in an ice bath, and collect the supernatant by centrifugation, wh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com