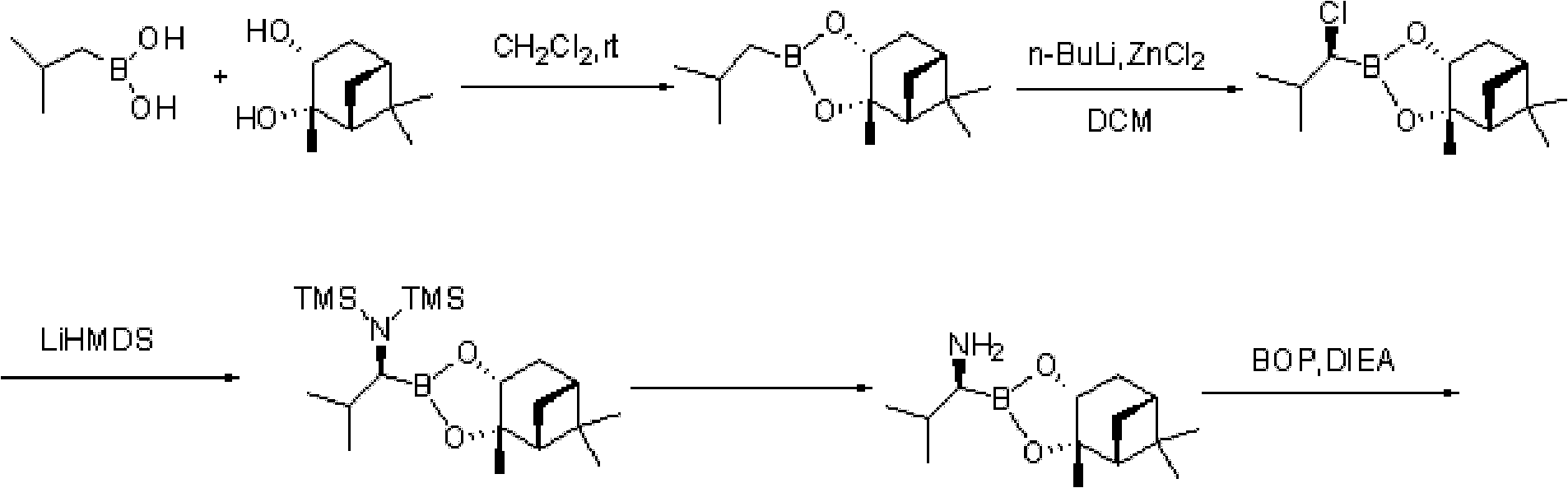
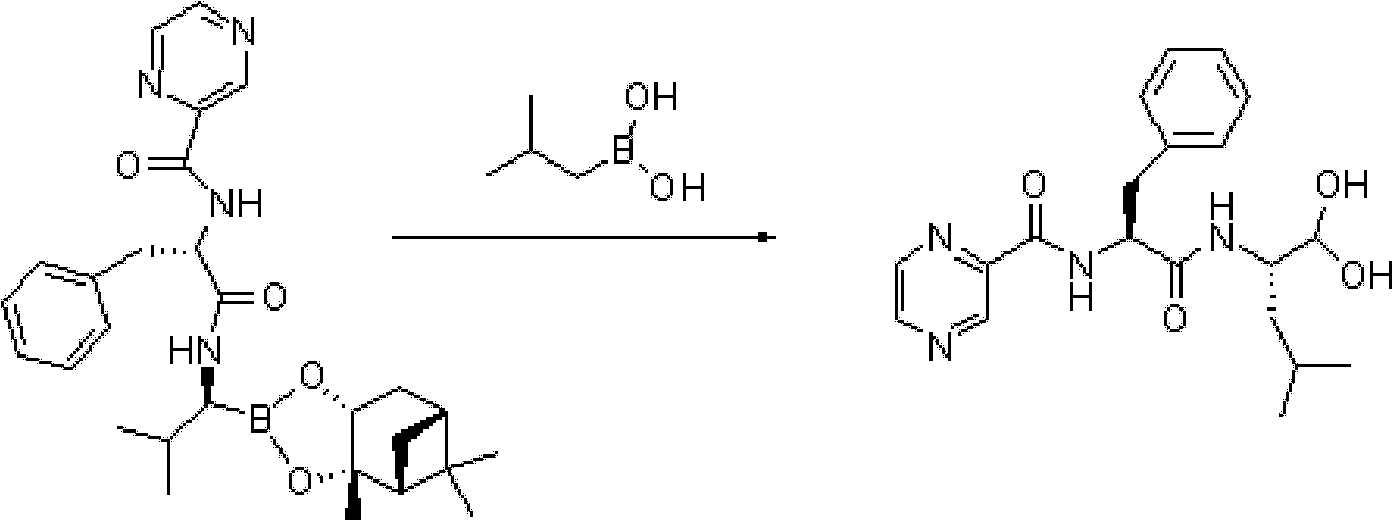
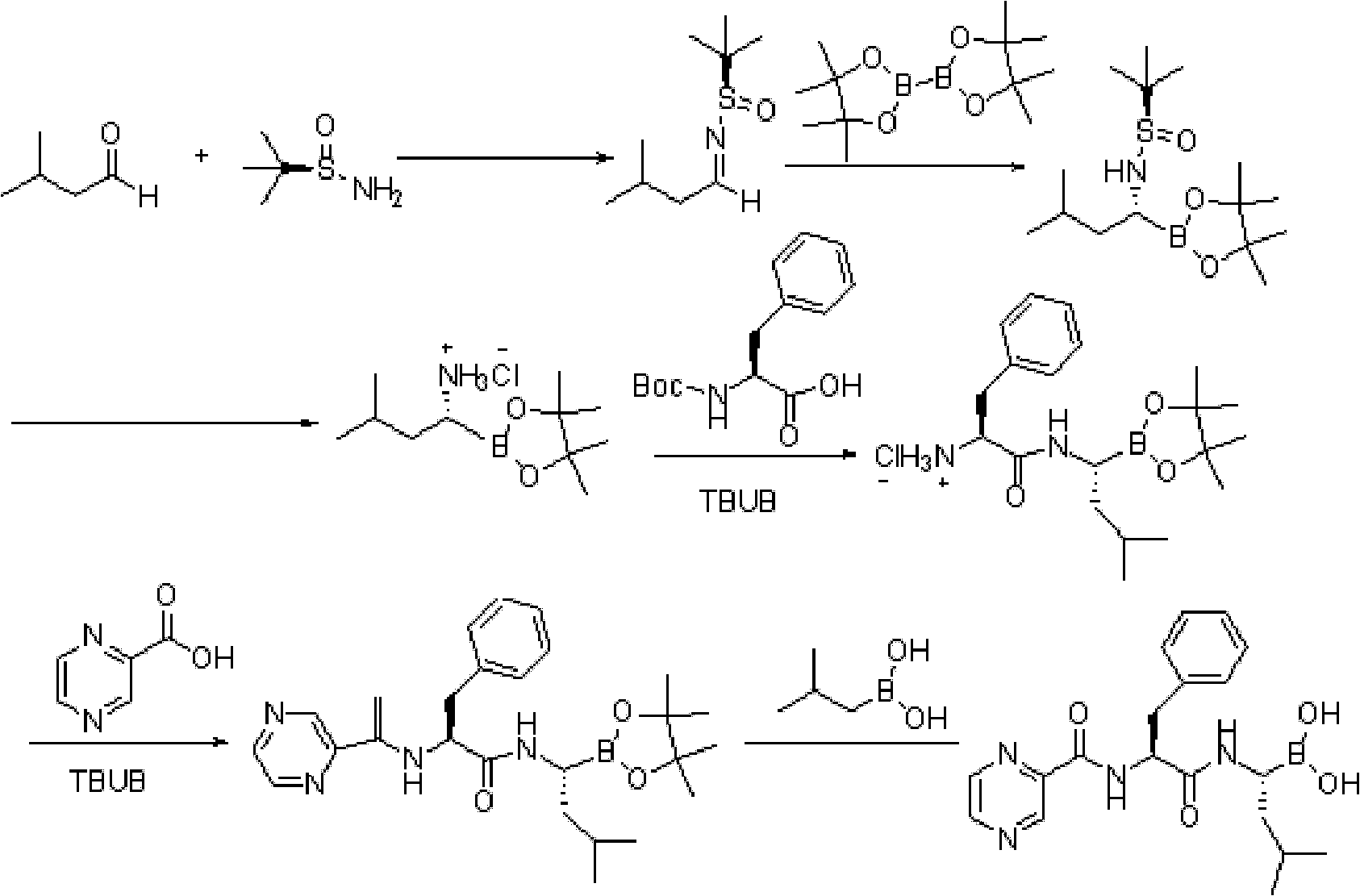
Method for preparing intermediate used for synthesizing bortezomib
A technology for bortezomib and intermediates, applied in the field of organic chemical synthesis, can solve problems such as difficulty in obtaining high optical purity bortezomib, inability to meet large-scale production requirements, and difficulty in obtaining raw materials, and achieve remarkable effects and economic practicability. , mild reaction conditions, simple post-processing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: Synthesis of (1-hydroxyl-3-methyl) butyl-pinacol borate III
[0036]
[0037] Add 43g (0.5mol) of 3-methylbutyraldehyde I, 2.5g (0.025mol) of cuprous chloride and 254g (1mol) of double pinacol borate II into a 1L four-necked flask, add 750ml of benzene, and reflux After reacting for 4 hours, the solvent was evaporated to dryness under reduced pressure to obtain 100.6 g of oily crude product III with a molar yield of 94%. The product was directly put into the next reaction without purification.
Embodiment 2
[0038] Embodiment 2: Synthesis of (1-hydroxyl-3-methyl) butyl-pinacol borate III
[0039]
[0040] Add 43g (0.5mol) 3-methylbutyraldehyde I, 5g (0.025mol) copper acetate and 381g (1.5mol) double pinacol borate II into a 2L four-necked flask, add 1L dichloromethane, and reflux After reacting for 6 hours, the solvent was evaporated to dryness under reduced pressure to obtain 96.5 g of oily crude product III with a molar yield of 90%, which was directly put into the next reaction without purification.
Embodiment 3
[0041] Embodiment 3: Synthesis of (1-hydroxyl-3-methyl) butyl-pinacol borate III
[0042]
[0043] Add 43g (0.5mol) 3-methylbutyraldehyde I, 3.6g (0.025mol) cuprous bromide and 191g (0.75mol) double pinacol borate II into a 1L four-necked flask, add 600ml of benzene, The reaction was refluxed for 8 hours, and the solvent was evaporated to dryness under reduced pressure to obtain 94 g of oily crude product III with a molar yield of 88%. The product was directly put into the next reaction without purification.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com