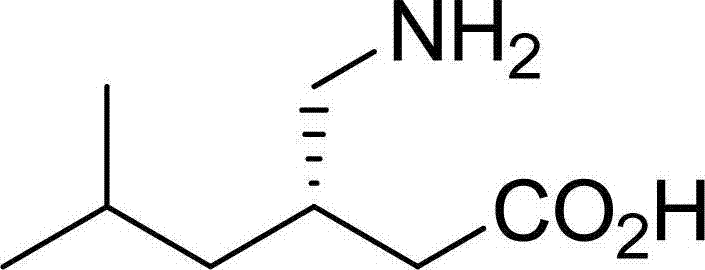
Method for preparing (3R)-(-)-3-(2- acetamino)-5-methylhexanol
A technology of acetylamino and methylhexanoic acid, which is applied in the field of medicine, can solve the problems of excessive waste water, waste of raw materials, and long reaction time, and achieve the effects of increased yield, reduced production cost, and shortened reaction cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
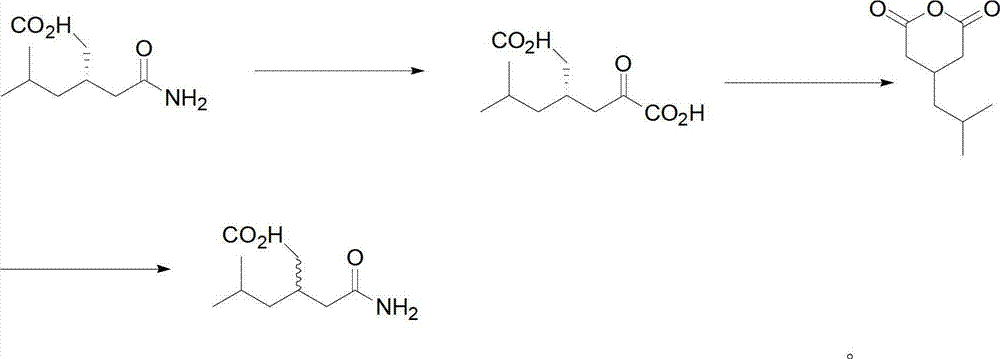
[0029] Example 1: Preparation of (3R)-(-)-3-(2-acetylamino)-5-methylhexanoic acid
[0030]
[0031] Put in 100g (±)-3-(2-acetylamino)-5-methylhexanoic acid racemate (prepared in Example 6), 2500g chloroform, 67.5g absolute ethanol, heat up to 55°C to dissolve, add 65g (S)-(-)-1-Phenylethylamine. Cool to 45°C, add 0.1g (3S)-(+)-3-(2-acetylamino)-5-methylhexanoic acid seed, cool to 25°C, filter with suction to get (3S)-(+)- 3-(2-Acetamido)-5-methylhexanoic acid phenethylamine salt 120g, set aside separately. The filtrate was concentrated to dryness under reduced pressure, 400 g of water and 2 g of activated carbon were added, stirred for decolorization, and suction filtered. The filtrate was adjusted to PH=1.5 with hydrochloric acid, cooled to 0~5°C, filtered with suction, and dried at 60°C to obtain 50 g of (3R)-(-)-3-(2-acetylamino)-5-methanol with an e.e. value of 90%. Hexanoic acid.
Embodiment 2
[0032] Example 2: Purification of (3R)-(-)-3-(2-acetylamino)-5-methylhexanoic acid
[0033]
[0034] 45g of (3R)-(-)-3-(2-acetylamino)-5-methylhexanoic acid (prepared in Example 1) with an e.e. value of 90% and 100g of absolute ethanol were dissolved at 60°C and cooled to Add 0.1g (3R)-(-)-3-(2-acetylamino)-5-methylhexanoic acid seed crystal at 45°C, cool at 5-10°C for 2 hours, filter with suction, and dry to obtain 35g (3R )-(-)-3-(2-acetylamino)-5-methylhexanoic acid, e.e. value 99.5%.
Embodiment 3
[0035] Embodiment 3: Preparation of 4-isobutyl-2,6-piperidone
[0036]
[0037] (3S)-(+)-3-(2-Acetamido)-5-methylhexanoic acid (prepared in Example 1) 200g, urea 60g, heat up to 160~180°C, and keep warm for 1 hour. Cool, add 400g of 60°C hot water at 80°C, cool to 0~5°C, and filter with suction to obtain 250g of 4-isobutyl-2,6-piperidone.
[0038] The test confirmed that this product is 4-isobutyl-2,6-piperidone. mp 136~138.3°C [Literature (Belsain database): 138~138.5°C]. MS (ESI): 168.9 (M+H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com