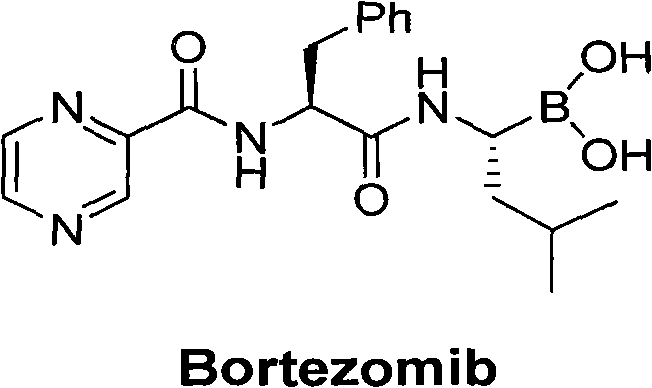
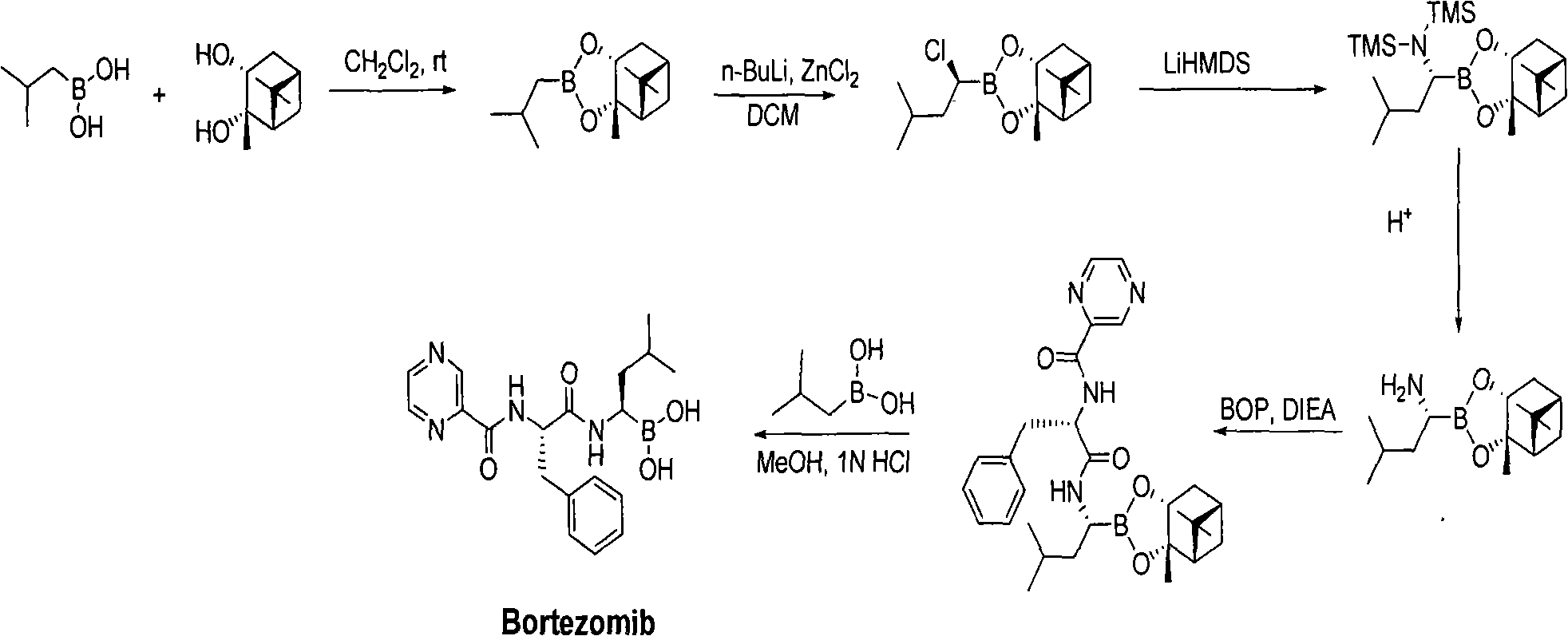
Method for synthesizing bortezomib
A synthesis method and bortezomib technology, applied in the direction of organic chemistry and the like, can solve the problems of harsh conditions, high cost and low yield, and achieve the effects of mild reaction conditions, low production cost and high route yield.
Active Publication Date: 2010-08-25
CHANGZHOU YABANG PHARMA
View PDF5 Cites 20 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
The current patent route uses 2-methylpropaneboronic acid as a raw material, and (1S, 2S, 3R, 5S)-(+)-2,3-pinanediol is condensed as a chiral ligand to form a borate, and then in anhydrous Under the catalysis of zinc dichloride, the insertion reaction of chloromethylene is carried out, followed by nucleophilic substitution of amine group, detrimethylsilyl group, then coupling with amino acid, and finally coupling with piperazine acid, through six-step synthesis Bortezomib, because (1S, 2S, 3R, 5S)-(+)-2,3-pinanediol is more expensive, there is no large-scale production in China; and the second step anhydrous zinc dichloride catalyzed chlorine The methyl insertion reaction needs to be carried out at minus 78 degrees, the conditions are harsh, and the reaction molar yield is low (only more than 50%); the third step of ditrimethylsilylamine is expensive, and the reaction also needs to be carried out at minus 78 degrees
Therefore, the raw materials of this route are expensive, the yield is low, and the synthesis conditions are harsh, which is not conducive to industrial operation.
Therefore, the cost of this route is relatively high, and it is not suitable for industrialized production.
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention belongs to the field of synthesizing medicaments, and discloses a method for synthesizing bortezomib. In the method, 3-methyl butyraldehyde and R-(+)-1-phenylethylamine are used as initiative materials, and the [(1R)-3-methyl-1-[[(2S)-1-oxygen-3-phenyl-2[(pyrazine formyl) amino] propyl]amino]butyl]-boric acid is obtained by condensation, selective boric acid ester addition, hydrogenation deprotection, chiral condensation with L-phenylalanine, condensation with 2-carboxyl-piperazine and boric acidification. The synthesis method has the advantages of readily available raw materials, higher yield of the whole reaction route, mild reaction conditions, easy operation, lower production cost and the suitability for industrialized production.
Description
technical field The invention belongs to the field of drug synthesis and relates to a synthesis method of bortezomib. Specifically pertaining to [(1R)-3-methyl-1-[[(2S)-1-oxo-3-phenyl-2-[(pyrazinecarbonyl)amino]propyl]amino]butyl]-boronic acid (Bortezomib, Bortezomib) synthetic method. Background technique Bortezomib (trade name: Velcade) is the first new drug approved for the treatment of multiple myeloma in the past ten years, and it is also the first cancer drug that targets protein-degrading enzyme complexes. About its mechanism of action His research won the Nobel Prize in Chemistry in 2004. Velcade Bortezomib was first discovered in early clinical trials in patients with multiple myeloma relapse and ineffective to other therapies. Since phase II clinical trials can significantly improve the condition of patients, it has been approved by the U.S. Food and Drug Administration (FDA) quickly. It was approved and officially launched in May 2003. On April 26, 2004, Europ...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07D241/24
Inventor 孙江涛孔立
Owner CHANGZHOU YABANG PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com