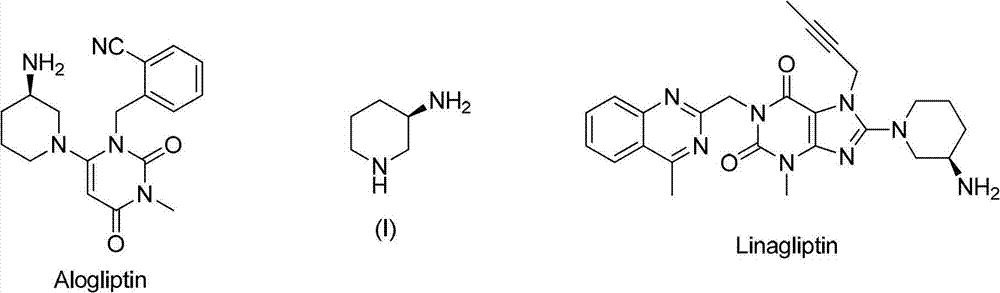
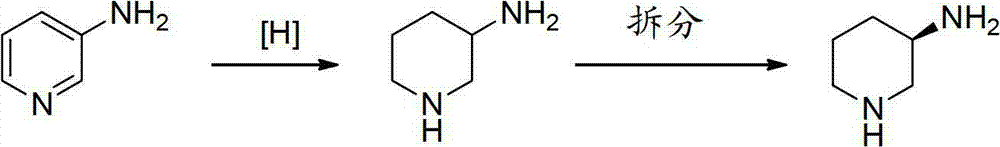
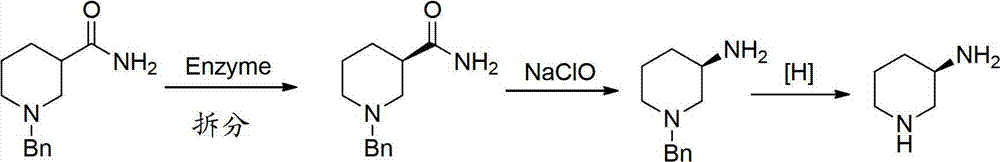
Asymmetric syntheses method and correlated intermediate of (R)-3-aminopiperidine (I)
A technology of aminopiperidine and synthesis method, applied in the production of bulk chemicals, organic chemistry, etc., can solve the problems of increasing industrial production cost and high price of D-ornithine, and achieve the effect of improving de value and simple process operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Preparation of (R)-3-((R)-1-phenylethyl)aminopiperidine-1-carboxylic acid tert-butyl ester (1-3)
[0035]
[0036] Under nitrogen protection, 10.0 g of 1-tert-butoxycarbonylpiperidin-3-one (1-1) (Changzhou Daou Chemical Co., Ltd.) and R-(+)-α-methylbenzylamine (1-2 ) (Guangde Keyuan Chemical Co., Ltd.) 7.3g was added to 60mL ethanol, and refluxed at 80°C for 3 hours. After the reaction is completed, the temperature is lowered to room temperature, 1.0 g of Ranny-Ni (Raney nickel) is added to the system, and the reaction is carried out at room temperature under a pressure of 30 kg for 48 hours. After the reaction is completed, filter with a sand core funnel with diatomaceous earth, spin the filtrate, and purify by column chromatography to obtain a light yellow liquid (R)-3-((R)-1-phenylethyl)aminopiperidine-1 - tert-butyl carboxylate (1-3) 12.4 g, de value: 93.2%. The obtained compound (1-3) was salted and crystallized with oxalic acid to obtain 13.7 g of ...
Embodiment 2
[0037] Example 2: Preparation of (R)-1-benzyl-N-((R)-1-phenylethyl)piperidin-3-amine (2-2)
[0038]
[0039]Under nitrogen protection, 5.0 g of 1-benzylpiperidin-3-one (2-1) (Changzhou Daou Chemical Co., Ltd.) and R-(+)-α-methylbenzylamine (1-2) ( Guangde Keyuan Chemical Co., Ltd.) 4.0g was added to 50mL benzene, refluxed at 80°C for 3 hours to separate water. After the reaction was completed, the temperature was lowered to room temperature, 1.0 g of palladium carbon was added to the system, and 40 kg of pressure was reacted at room temperature for 48 hours. After the reaction is completed, filter with a sand core funnel lined with diatomaceous earth, spin the filtrate, and purify by column chromatography to obtain a light yellow liquid (R)-1-benzyl-N-((R)-1-phenylethyl) Piperidin-3-amine (2-2) 6.4 g, de value: 35%. The obtained compound (2-2) was salted and crystallized with oxalic acid to obtain 4.5 g of white oxalate crystals, the total yield: 44.3%, de value: 98.4%. ...
Embodiment 3
[0040] Example 3: Preparation of (R)-tert-butyl 3-aminopiperidine-1-carboxylate (3-1)
[0041]
[0042] Add (R)-3-((R)-1-phenylethyl)aminopiperidine-1-carboxylate tert-butyl ester (1-3) 10.0g and Pd / C 1.0g to 40mL ethanol and 40mL acetic acid In the mixed solution, 30 kilograms of pressures were reacted at room temperature for 48 hours. After the reaction was completed, filter with a sand core funnel lined with diatomaceous earth, and spin the filtrate to obtain 5.4 g of (R)-tert-butyl 3-aminopiperidine-1-carboxylate (3-1) as a light yellow viscous liquid. Yield: 82.1%, the product is directly used in the next step. 1 H NMR (400MHz, CDCl 3 )δ7.96(s,6H),4.03(d,J=11.6Hz,1H),3.74(d,J=6.7Hz,1H),3.12(s,1H),3.04-2.84(m,2H), 1.73(s,1H),1.56(dd,J=24.6,11.9Hz,2H),1.45(s,9H),1.36-1.16(m,2H).MS(ESI)m / z=201(M + +1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com