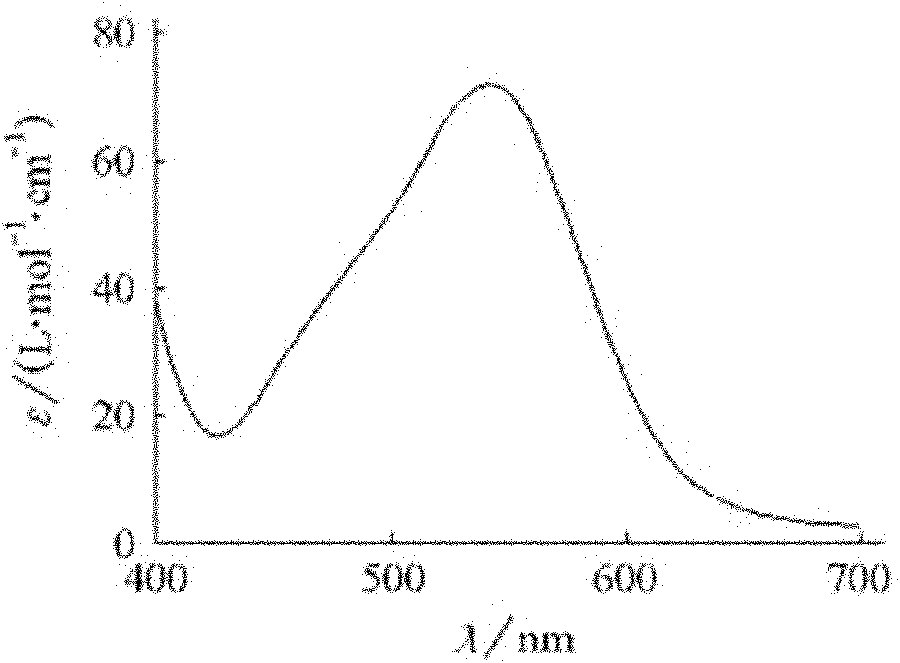
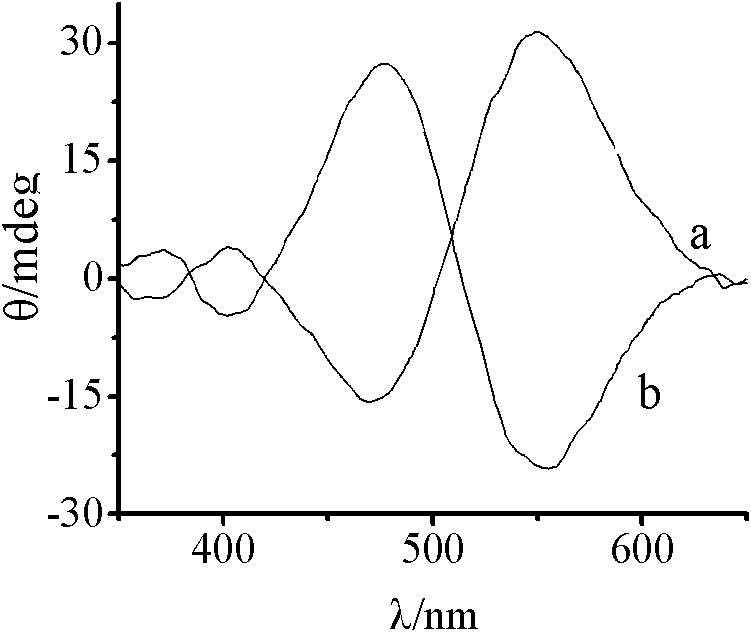
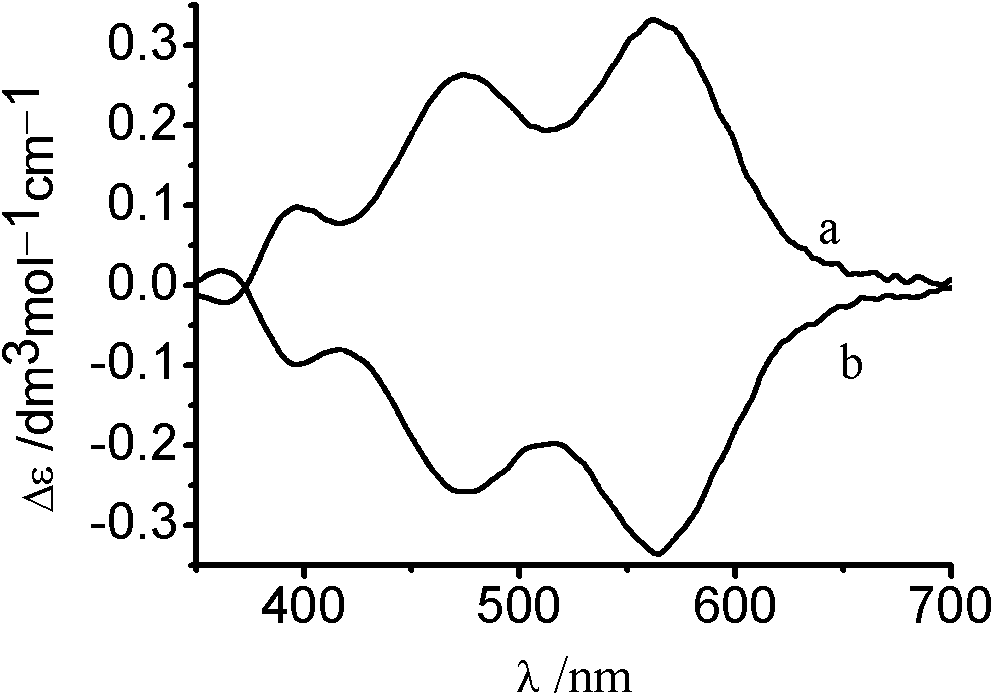
Synthesis method of chiral cis-bromo.ammine.bis(ethylenediamine) cobalt bromide
A synthesis method, ethylenediamine technology, applied in organic chemistry methods, chemical instruments and methods, asymmetric synthesis, etc., can solve problems such as chiral symmetry breaking, and achieve easy storage, good safety, and easy control Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Will [Co(H 2 O) 2 {(μ-OH) 2 Co(en) 2} 2 ](SO 4 ) 2 ·7H 2 O (0.72g), NH 4 Br (2g) and 2g of water were mixed and stirred magnetically for 1min to form a suspension, and 0.0036gΛ-cis-[CoBr(NH 3 )(en) 2 ]Br 2 variety, then heated in a 50°C water bath and stirred for 5 minutes, and kept in a refrigerator (4°C) for more than 20 hours after cooling to obtain more crystals. The precipitated red-purple crystalline solid was filtered and washed successively with ethanol and ether to remove ammonium bromide and other by-products until no turbid liquid was washed out. The crude product was recrystallized with a minimum amount of 5% hydrobromic acid solution, and the saturated solution was left to crystallize at room temperature to obtain the compound Λ-cis-[CoBr(NH 3 )(en) 2 ]Br 2 . Yield 61.8%, Δε=0.354.
Embodiment 2
[0021] Will [Co(H 2 O) 2 {(μ-OH) 2 Co(en) 2} 2 ](SO 4 ) 2 ·7H 2 O (0.72g), NH 4 Br (2g) and 2g of water were mixed and stirred magnetically for 1min to form a suspension, and 0.0036g of Δ-cis-[CoBr(NH 3 )(en) 2 ]Br 2 variety, then heated in a 50°C water bath and stirred for 5 minutes, and kept in a refrigerator (4°C) for more than 20 hours after cooling to obtain more crystals. The precipitated red-purple crystalline solid was filtered and washed successively with ethanol and ether to remove ammonium bromide and other by-products until no turbid liquid was washed out. The crude product was recrystallized with a minimum amount of 5% hydrobromic acid solution, and the saturated solution was left to crystallize at room temperature to obtain a compound Δ-cis-[CoBr(NH 3 )(en) 2 ]Br 2. Yield 60.4%, Δε=-0.347.
Embodiment 3
[0023] Will [Co(H 2 O) 2 {(μ-OH) 2 Co(en) 2} 2 ](SO 4 ) 2 ·7H 2 O (2.14g), NH 4 Br (6.86g) and 6.8g of water were mixed and stirred magnetically for 1min to form a suspension, and 0.0107g of Δ-cis-[CoBr(NH 3 )(en) 2 ]Br 2 variety, then heated in a 50°C water bath and stirred for 5 minutes, and kept in a refrigerator (4°C) for more than 20 hours after cooling to obtain more crystals. The precipitated red-purple crystalline solid was filtered and washed successively with ethanol and ether to remove ammonium bromide and other by-products until no turbid liquid was washed out. The crude product was recrystallized with a minimum amount of 5% hydrobromic acid solution, and the saturated solution was left to crystallize at room temperature to obtain a compound Δ-cis-[CoBr(NH 3 )(en) 2 ]Br 2 . Yield 50.6%, Δε=-0.327.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com