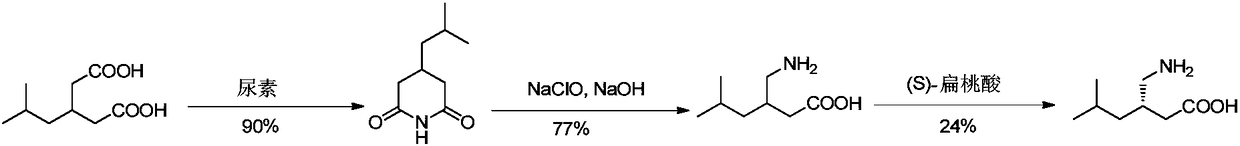
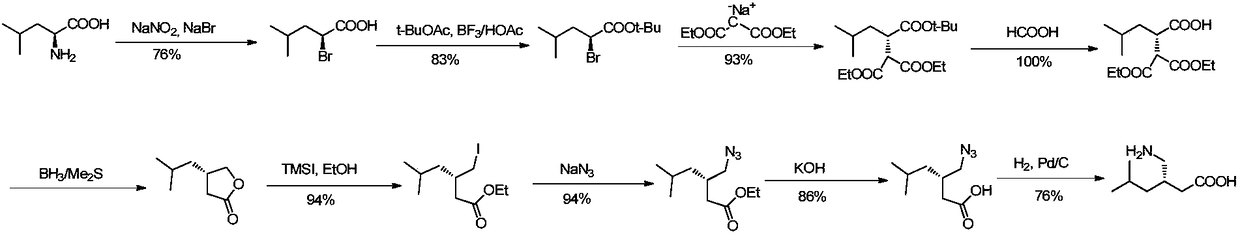
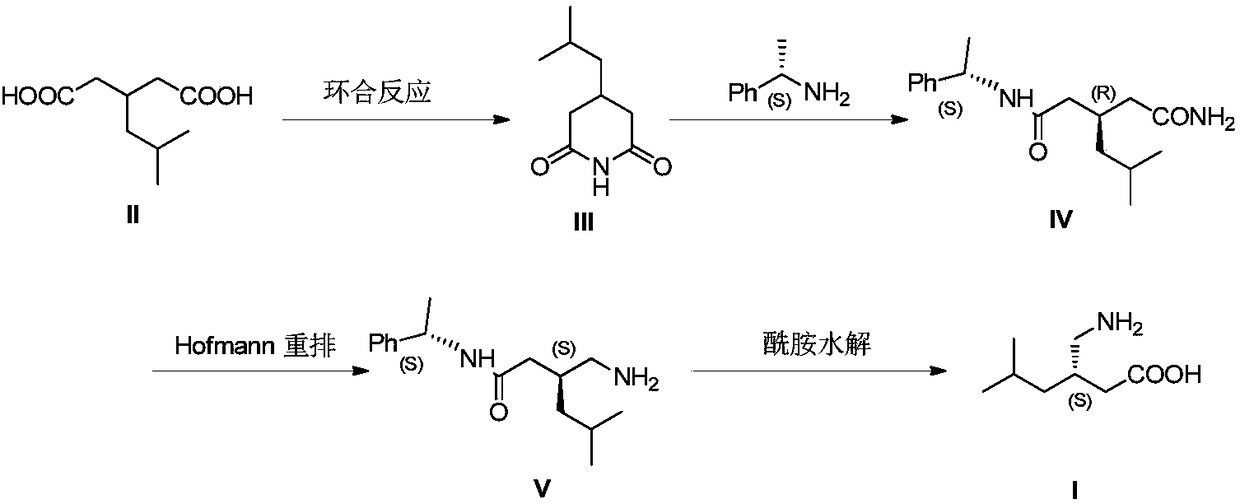
Method for asymmetric preparation of (S)-3-aminomethyl-5-methylcaproic acid
A methyl hexanoic acid, asymmetric technology, applied in the field of chemical medicine, can solve the problems of difficult recovery and recycling, hindered industrial production, expensive catalysts, etc., and achieve the effect of cheap raw materials, short reaction steps and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The synthesis of embodiment 1 (S)-3-aminomethyl-5-methylhexanoic acid
[0029] (1) Preparation of compound III
[0030] Add 3-isobutylglutaric acid (1kg) and urea (0.64kg) in the 5L round bottom flask, react the reaction mixture at 180 ℃ for 2 hours, cool to 80 ℃, add water (1L), ethanol (2L), Activated carbon (50 g), heated at 80° C. for 0.5 hour, filtered hot, cooled to crystallize, filtered, and dried to obtain white crystalline compound III (0.83 kg, yield: 92%).
[0031] (2) Preparation of Compound IV
[0032] Toluene (2.7L), (S)-(+)-1-phenethylamine (0.88kg) and DMAP (5.9g) were added in a 6L round bottom flask, and compound III (0.83 kg) were added to the flask in batches (the addition was completed in 1 hour), and the reaction was stirred at -20 to -10°C for 3 hours. The reaction solution was washed with water, dried, concentrated under reduced pressure, and then crystallized with toluene to obtain compound IV (1.42 kg, yield: 90%), with ee of 99.4%.
[0033...
Embodiment 2
[0037] The synthesis of embodiment 2 (S)-3-aminomethyl-5-methylhexanoic acid
[0038] (1) Preparation of compound III
[0039] Add 3-isobutylglutaric acid (1kg) and 40% ammonia water (0.68kg) in the 5L round bottom flask, react the reaction mixture at 100 ℃ for 2 hours, cool to 80 ℃, add water (1L), ethanol (2L ), activated carbon (50 g), heated at 80° C. for 0.5 hour, hot filtered, cooled to crystallize, filtered, and dried to obtain white crystalline compound III (0.81 kg, yield: 90%).
[0040] (2) Preparation of Compound IV
[0041] Toluene (2.7L), (S)-(+)-1-phenethylamine (0.88kg) and DMAP (5.9g) were added in a 6L round bottom flask, and compound III (0.81 kg) were added to the flask in batches (the addition was completed in 1 hour), and the reaction was stirred at -20 to -10°C for 3 hours. The reaction solution was washed with water, dried, concentrated under reduced pressure, and then crystallized with toluene to obtain compound IV (1.39 kg, yield: 90%), with ee of 9...
Embodiment 3
[0046] The synthesis of embodiment 3 (S)-3-aminomethyl-5-methylhexanoic acid
[0047] (1) Preparation of compound III
[0048]Add 3-isobutylglutaric acid (1kg) and ammonium bicarbonate (1.26kg) to a 5L round bottom flask, react the reaction mixture at 180°C for 2 hours, cool to 90°C, add water (1L), ethanol (2L ), activated carbon (50 g), heated at 80° C. for 0.5 hour, hot filtered, cooled to crystallize, filtered, and dried to obtain white crystalline compound III (0.82 kg, yield: 91%).
[0049] (2) Preparation of compound IV
[0050] Toluene (2.7L), (S)-(+)-1-phenethylamine (0.88kg) and DMAP (5.9g) were added in a 6L round bottom flask, and compound III (0.82 kg) into the flask in batches (the addition was completed in 1 hour), and the reaction was stirred at -20 to -10°C for 3 hours. The reaction solution was washed with water, dried, concentrated under reduced pressure, and then crystallized with toluene to obtain compound IV (1.45 kg, yield: 93%), with ee of 99.1%.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com