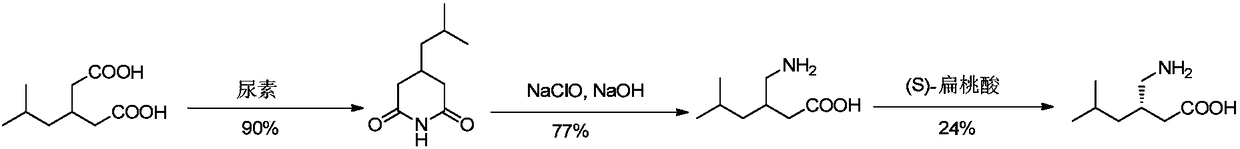
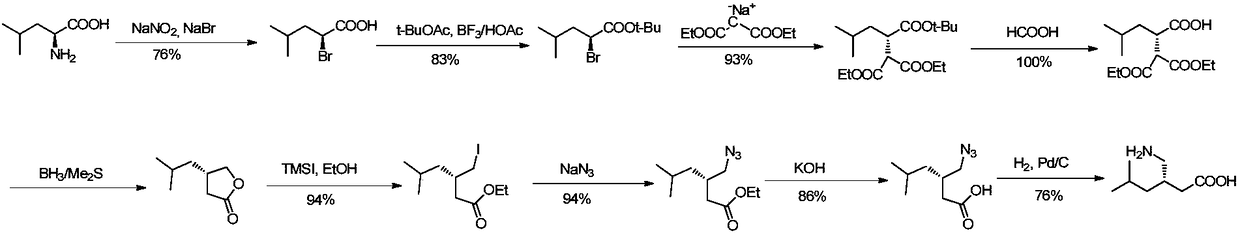
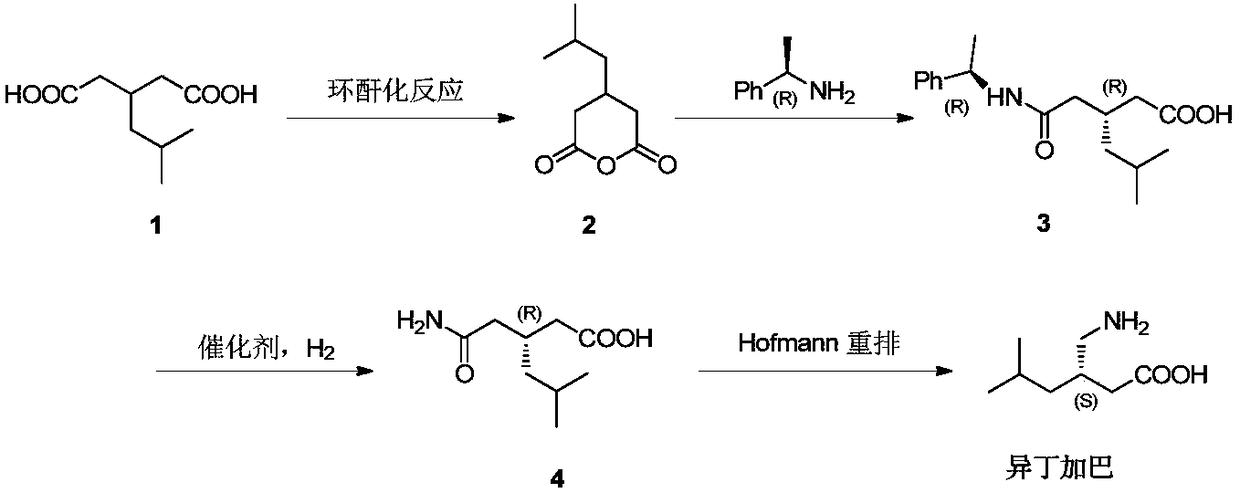
Asymmetrical synthesis method of lyrica
A technique of isobutylcarba and its synthesis method, which is applied in the field of chemistry and medicine, can solve the problems that chiral catalysts are expensive and not suitable for large-scale production, and achieve the effect of cheap raw materials, few reaction steps, and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The synthesis of embodiment 1 ibugaba
[0031] (1) Preparation of compound 2
[0032] Add 3-isobutylglutaric acid (1kg) and acetic anhydride (0.66kg) into a 1.5L round-bottomed flask, reflux the reaction mixture at 135-145°C for 2.5-3 hours, and evaporate unreacted acetic anhydride under vacuum , and cooled to obtain 0.91kg of compound 2, which was directly carried out to the next step without purification.
[0033] (2) Preparation of compound 3
[0034] Add toluene (3L), (R)-(+)-1-phenethylamine (0.97kg) and DMAP (6.5g) in 6L round-bottomed flask, compound 2 (0.91kg) at -20~-10 ℃ ) in toluene (1.5L) was slowly added into the flask (1-1.5h was added), and the reaction was stirred at -20~-10°C for 3-5 hours. The reaction solution was extracted with NaOH aqueous solution, the aqueous phase was washed with toluene, the pH value was adjusted to 2 by adding hydrochloric acid (2N) aqueous solution, the aqueous phase was extracted with ethyl acetate, dried, concentrated und...
Embodiment 2
[0039] The synthesis of embodiment 2 Ibugaba
[0040] (1) Preparation of Compound 2
[0041] Add 3-isobutylglutaric acid (10kg) and acetic anhydride (6.6kg) into a 15L reactor, and the reaction mixture is refluxed at 135-145°C for 4-6 hours, and the unreacted acetic anhydride is evaporated under vacuum, cooled 9.0kg of compound 2 was obtained, which was directly carried out to the next step without purification.
[0042] (2) Preparation of Compound 3
[0043] Add toluene (30L), (R)-(+)-1-phenethylamine (9.7kg) and DMAP (65g) in 60L reaction kettle, under -20~-10 ℃, compound 2 (9.0kg) The toluene solution (15L) was slowly added into the flask (3-3.5h to complete the addition), and the reaction was stirred at -20~-10°C for 5-6 hours. The reaction solution was extracted with NaOH aqueous solution, the aqueous phase was washed with toluene, the pH value was adjusted to 2 by adding hydrochloric acid (2N) aqueous solution, the aqueous phase was extracted with ethyl acetate, dried...
Embodiment 3
[0048] The synthesis of embodiment 3 Ibugaba
[0049] (1) Preparation of Compound 2
[0050] Add 3-isobutylglutaric acid (1kg) and acetic anhydride (0.75kg) in the 1.5L round-bottomed flask, the reaction mixture was refluxed for 2 hours at 51°C, evaporate unreacted acetic anhydride under vacuum, and cool to obtain 0.85 kg of compound 2 was directly carried out to the next step without purification.
[0051] (2) Preparation of Compound 3
[0052] Toluene (3L), (R)-(+)-1-phenethylamine (0.92kg) and DMAP (6.2g) were added in a 6L round bottom flask, and compound 2 (0.85kg ) in toluene (1.5L) was slowly added into the flask (1-1.5h was added), and the reaction was stirred at -20~-10°C for 3-5 hours. The reaction solution was extracted with NaOH aqueous solution, the aqueous phase was washed with toluene, the pH value was adjusted to 2 by adding hydrochloric acid (2N) aqueous solution, the aqueous phase was extracted with ethyl acetate, dried, concentrated under reduced pressure, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com