Catalytic rectification process and device for chiral resolution of 1-phenylethylamine by using lipase
A technology of phenylethylamine catalytic refinement and catalytic rectification tower, which is applied in the chemical industry, distillation separation, organic chemistry, etc., can solve problems such as difficult to obtain product conversion rate, and achieve reduced contact time, high reaction conversion rate, and optical purity high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
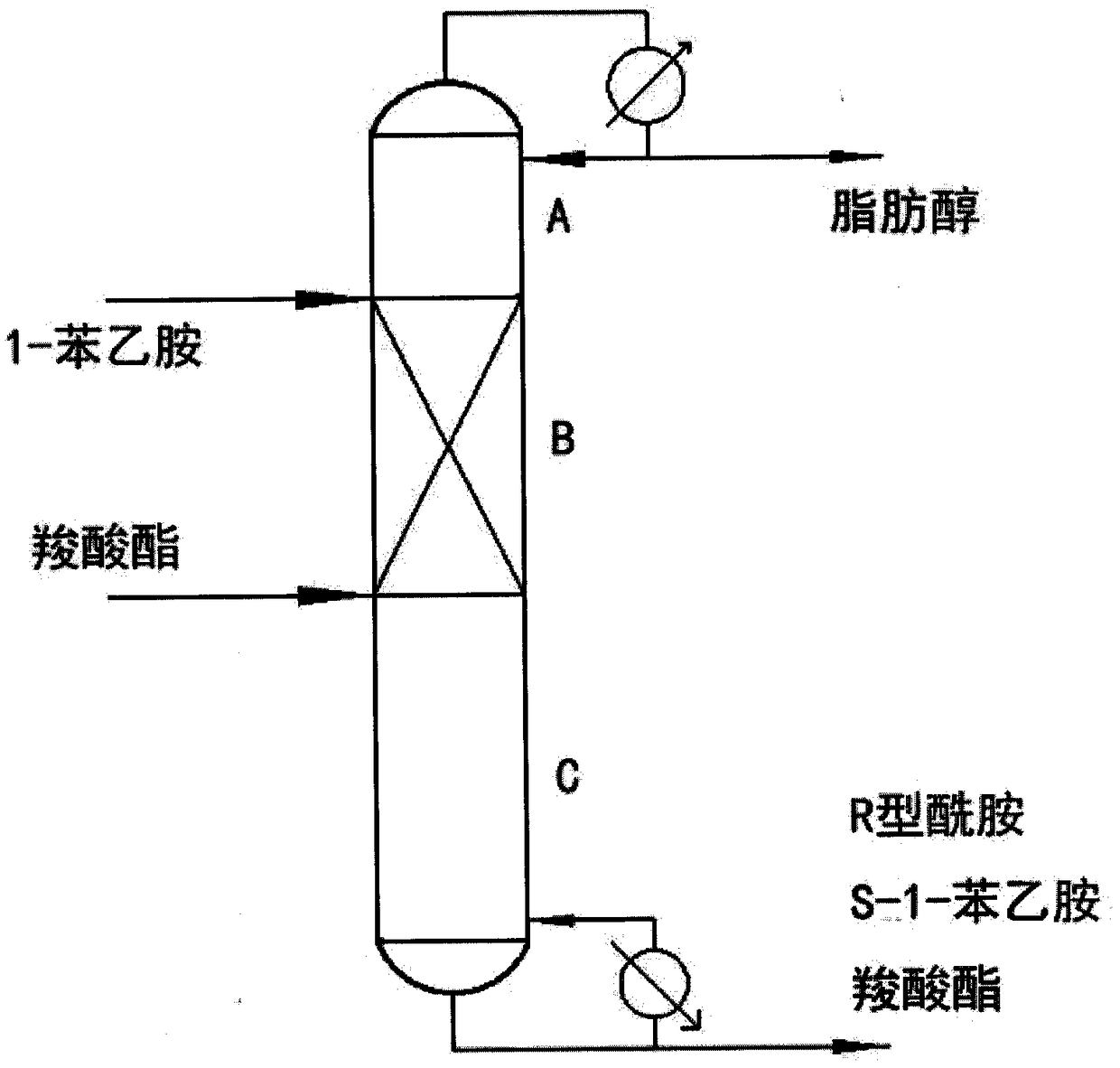
[0023] The operating pressure of the catalytic rectification column used was 300 mBar. 1-Phenylethylamine is continuously fed from the upper feed port of the reaction section after being preheated to 60°C. Ethyl butyrate is continuously fed from the feed port at the lower part of the reaction section after being preheated to 60°C. The molar ratio of feed 1-phenethylamine to ethyl butyrate is 1:3. 1-Phenylethylamine and ethyl butyrate react under the catalysis of the lipase catalyst loaded in the reaction section to generate R-amide and ethanol. The temperature of the tower kettle is 100-101°C, and the tower kettle reboiler provides the ascending gas phase for the upper sections. The top reflux ratio of the catalytic distillation tower is 3.4, and the temperature is 50-51°C. Ethanol is extracted from the top of the catalytic rectification tower, and R-amide and unreacted S-1-phenylethylamine and ethyl butyrate are extracted from the bottom of the tower.
[0024] After testin...
Embodiment 2
[0026] The operating pressure of the catalytic rectification column used was 500 mBar. 1-Phenylethylamine is continuously fed from the upper feed port of the reaction section after being preheated to 60°C. Methyl butyrate is fed continuously from the feed port at the lower part of the reaction section after being preheated to 60°C. The molar ratio of feed 1-phenylethylamine to methyl butyrate is 1:2. 1-Phenylethylamine and methyl butyrate react under the catalysis of the lipase catalyst loaded in the reaction section to generate R-amide and methanol. The temperature of the tower kettle is 98-99°C, and the tower kettle reboiler provides the rising gas phase for the upper sections. The top reflux ratio of the catalytic rectification tower is 2.2, the temperature is 47-48°C, and the methanol product is extracted from the top.
[0027] After testing, the conversion rate of R-1-phenylethylamine in the catalytic rectification tower is ≥99.5%, the mass fraction of methanol in the p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com