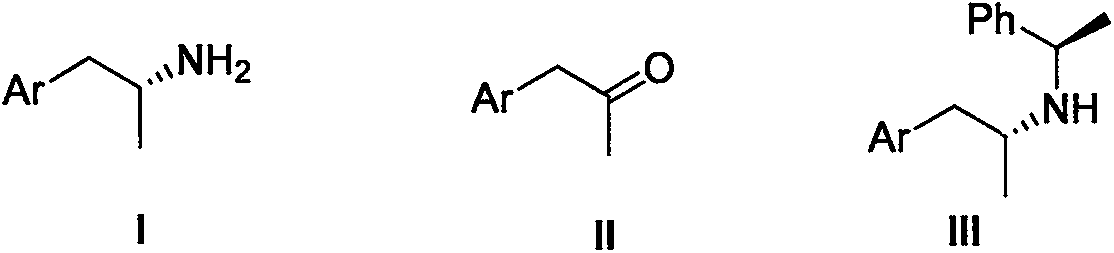
Improved synthetic method of (R)-1-aryl-2-propylamine
A synthetic method, the technology of aryl acetone, applied in the field of drug synthesis, can solve the problems of wasting raw materials, low yield, poor economy, etc., and achieve high application value and simple reaction operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024]
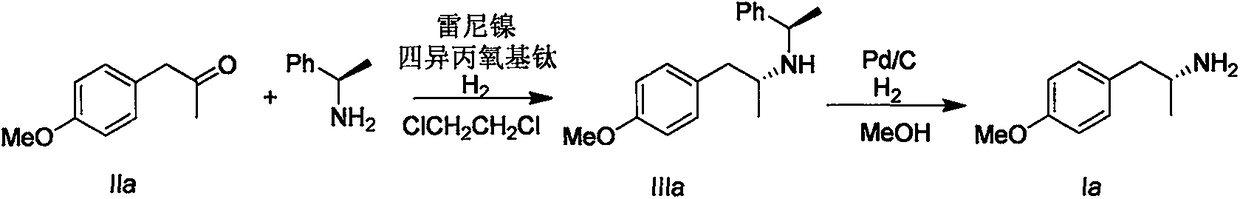
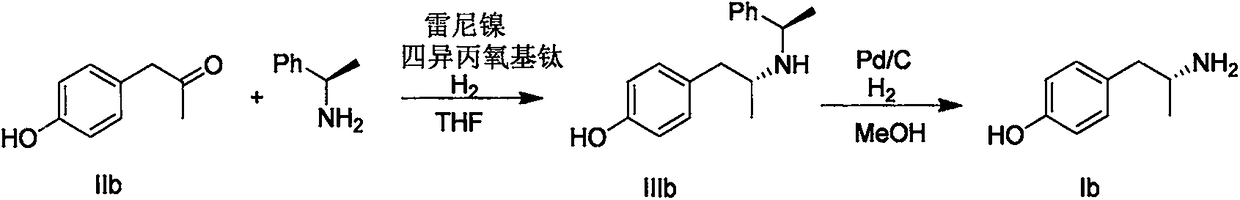
[0025] Add 1-(4-methoxyphenyl)-2-propanone IIa (16.4g, 100mmol), 500mL dichloroethane, (R)-1-phenyl-ethylamine (12.1g, 100mmol) and tetraisopropoxytitanium (34.1g, 120mmol), stirred at room temperature for 30 minutes after the addition, then added Raney nickel (16g), charged with 10 atmospheres of hydrogen, and in an oil bath at 45°C Stir overnight. The reaction solution was cooled and filtered, and dried over anhydrous magnesium sulfate. The solvent was removed under reduced pressure, and the residue was purified by silica gel column chromatography (eluent: dichloromethane / anhydrous methanol=50 / 1) to obtain IIIa (26.0 g, yield 96%).
[0026] Add IIIa (26.0g), 500mL methanol, and 10% palladium carbon (2g) into the autoclave, fill with 20 atmospheres of hydrogen, and react in an oil bath at 45°C for 8 hours. The reaction solution was cooled and filtered, and the solvent was removed under reduced pressure to obtain a white solid Ia (15 g, yield 94%, ee value 99.9%)...
Embodiment 2
[0028] Using the same procedure as in Example 1, boron trifluoride diethyl ether (17 g, 120 mmol) was used instead of titanium tetraisopropoxide to obtain product Ia (8.5 g, yield 51%, ee value 99.9%).
Embodiment 3
[0030] Using the same procedure as in Example 1, using zirconium tetrabutoxide (46 g, 120 mmol) instead of titanium tetraisopropoxide, the product Ia (15 g, yield 90%, ee value 99.9%) was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com