Method for synthesizing (1S)-4,5-dimethoxy-1-(carbonylaminomethyl)benzocyclobutane
A carbonylaminomethyl, benzocyclobutane technology, applied in the field of pharmaceutical synthesis, can solve the problems of high production cost, unsuitable for industrialized production, low total yield and the like, and achieves good product quality, easy recovery and application, and elimination. The effect of less spin pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
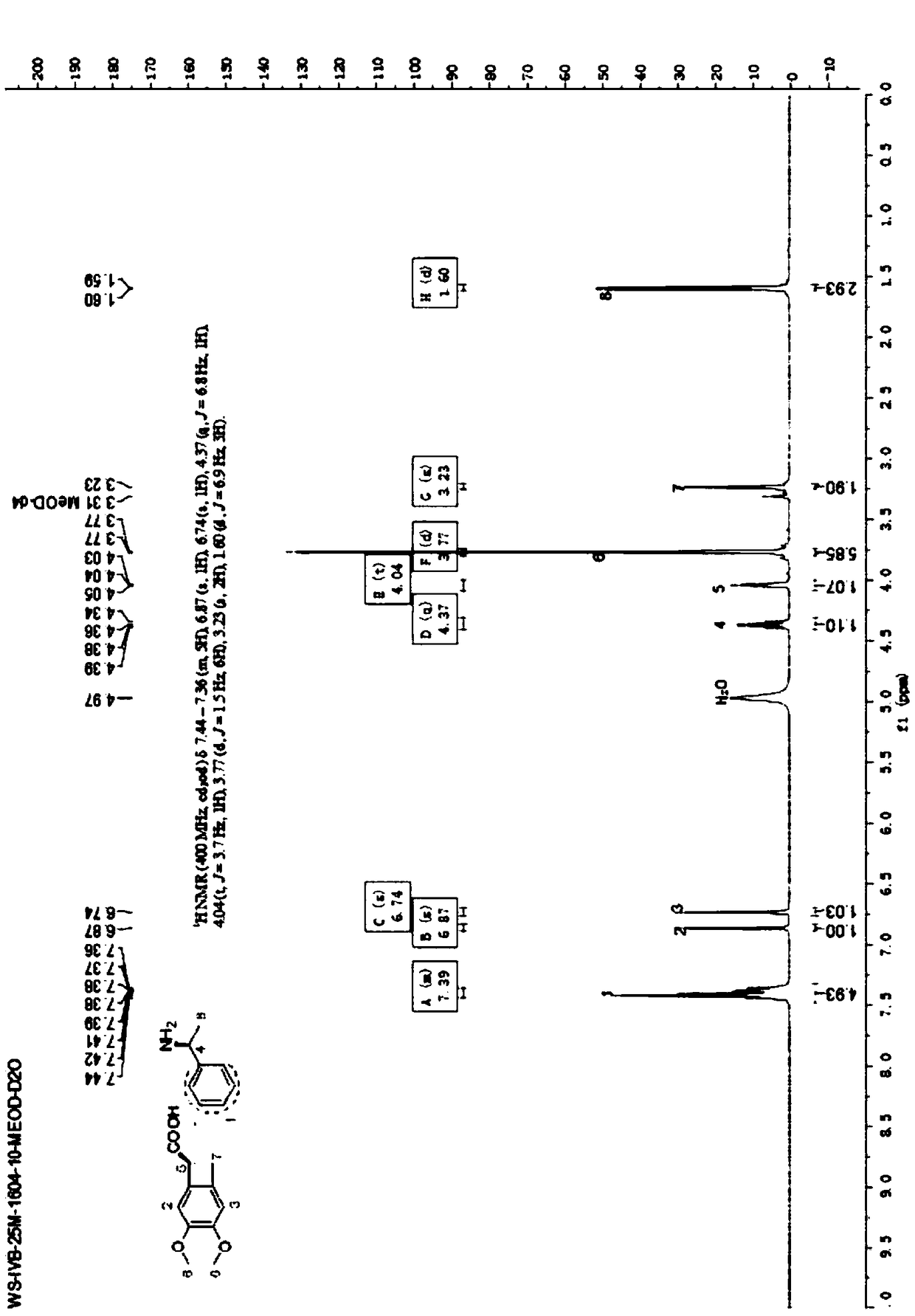
[0030] The synthesis method of (1S)-4,5-dimethoxy-1-(carbonylaminomethyl)benzocyclobutane in this embodiment includes the following steps:
[0031] (1) Put 500mL dichloromethane, 10mL absolute ethanol, 100g compound of formula 5 into the reaction flask, stir and slowly raise the temperature to 35℃, add 35g S-(-)-phenethylamine dropwise at 35℃, and keep the dripping process Slowly heat, and then continue to heat up and reflux for 30-60 minutes after the addition is completed (solids may be generated during the reflux process, and the stirring speed will be increased immediately);
[0032] After refluxing, cool to 15-20°C and keep stirring for 3 to 5 hours, filter, and wash the filter cake with a mixed solution of 100 mL of dichloromethane and 2 mL of ethanol, and retain the mother liquor;
[0033] Dry under reduced pressure at 40~45℃ for 8 hours, check the chiral purity by Chiral HPLC ≥97%, the dry weight of the collected material is 65.5g, the resolution yield is 41.3%, the compound ...
Embodiment 2
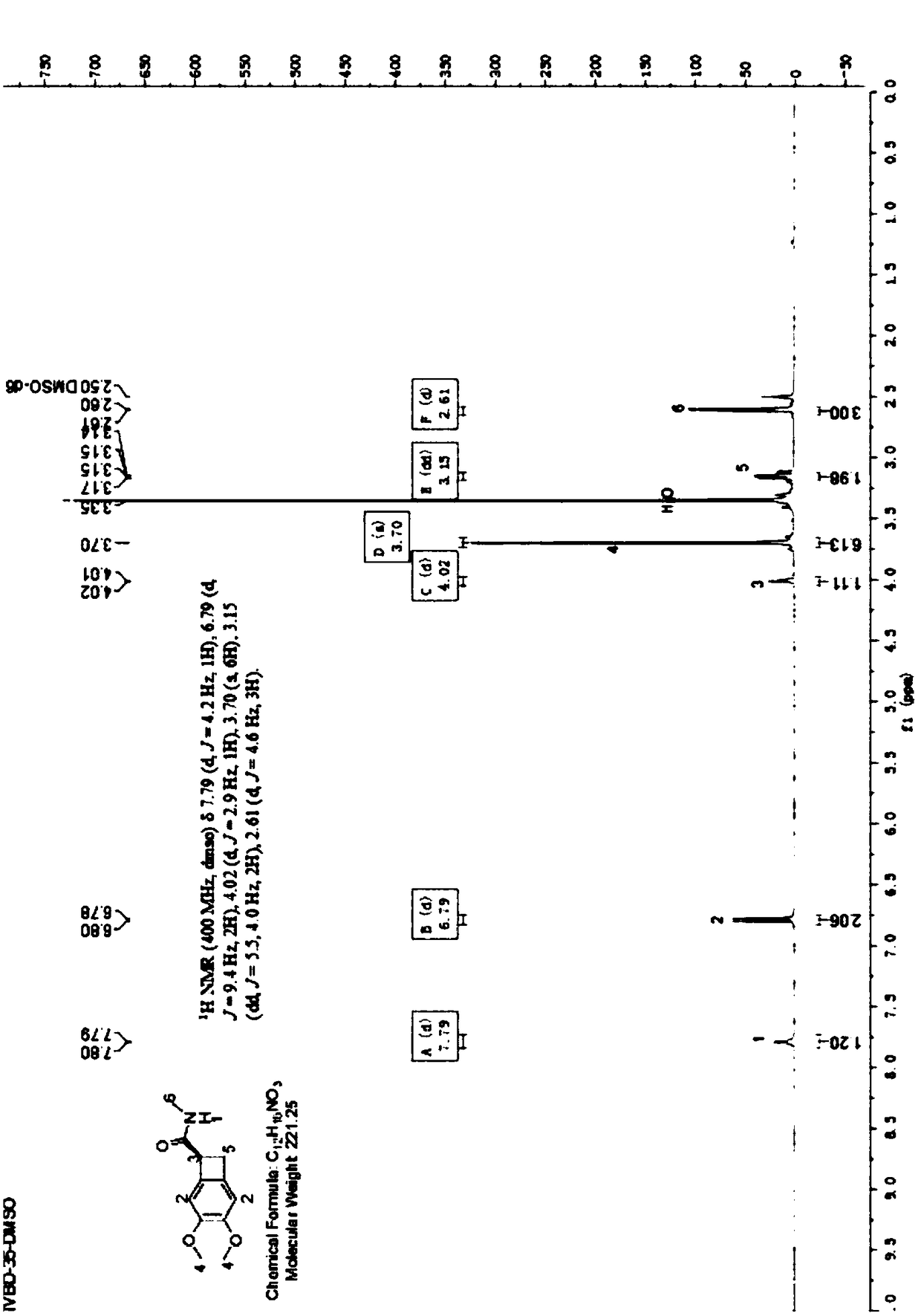
[0041] (1) Put 500mL chloroform, 20mL absolute ethanol, 100g compound of formula 5 into the reaction flask, stir and slowly raise the temperature to 35℃, add 32g S-(-)-phenethylamine dropwise at 35℃, and keep the dripping process Slowly heat, and then continue to heat and reflux for 30-60 minutes after the addition is complete;
[0042] After refluxing, cool to 15~20℃ and keep stirring for 3~5 hours, filter, wash the filter cake with 100mL chloroform, and keep the mother liquor;
[0043] Dry under reduced pressure at 40-45°C for 8 hours, check chiral purity by Chiral HPLC ≥97%, the dry weight of the collected material is 72.8g, and the resolution yield is 46%.
[0044] (2) Add the separated mother liquor to a 1000mL reaction flask, adjust the pH to less than 4 with 6N hydrochloric acid, stand still for layering to obtain an organic layer, wash the organic layer with 100mL water again, and then stand for layering to obtain an organic layer; concentrate under reduced pressure Obtain o...
Embodiment 3
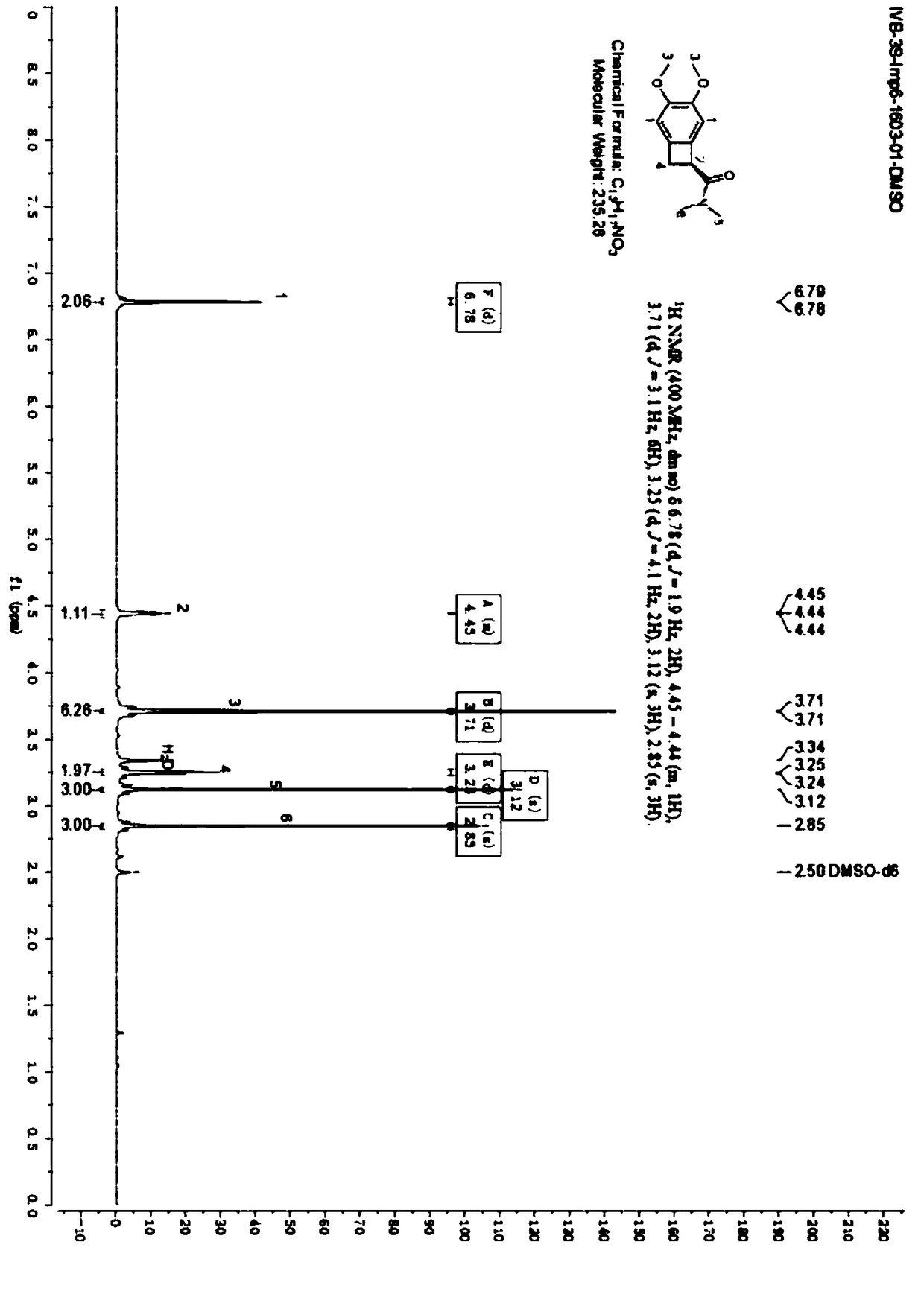
[0051] (1) Put 500mL 1,2-dichloroethane, 10mL absolute ethanol, 100g compound of formula 5 into the reaction flask, stir and slowly raise the temperature to 35℃, and add 40g S-(-)-phenethylamine dropwise at 35℃ , Keep heating slowly during the dripping process, and keep the temperature at 60℃ for 30-60 minutes after dripping;
[0052] After refluxing, cool to 15-20°C and keep stirring for 3 to 5 hours, filter, and wash the filter cake with a mixed solution of 100 mL 1,2-dichloroethane and 10 mL ethanol, and retain the mother liquor;
[0053] It was dried under reduced pressure at 40-45°C for 8 hours, the chiral purity was detected by Chiral HPLC ≥ 97%, the dry weight of the collected material was 75.9 g (the compound of formula 4), and the resolution yield was 48.1%.
[0054] (2) The resulting separated mother liquor was added to a 1000 mL reaction flask, adjusted to pH less than 2 with 6N hydrochloric acid, left to stand for layering to obtain an organic layer, the organic layer was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com