Chiral MOC liquid chromatography separation column for resolution of racemic compounds
A liquid chromatography column and liquid chromatography technology, applied in the field of chiral MOC liquid chromatography column, can solve the problems of pore window size, cavity size and shape chiral recognition ability, etc., and achieve good application prospects, separation The effect is good and the raw materials are simple and easy to obtain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
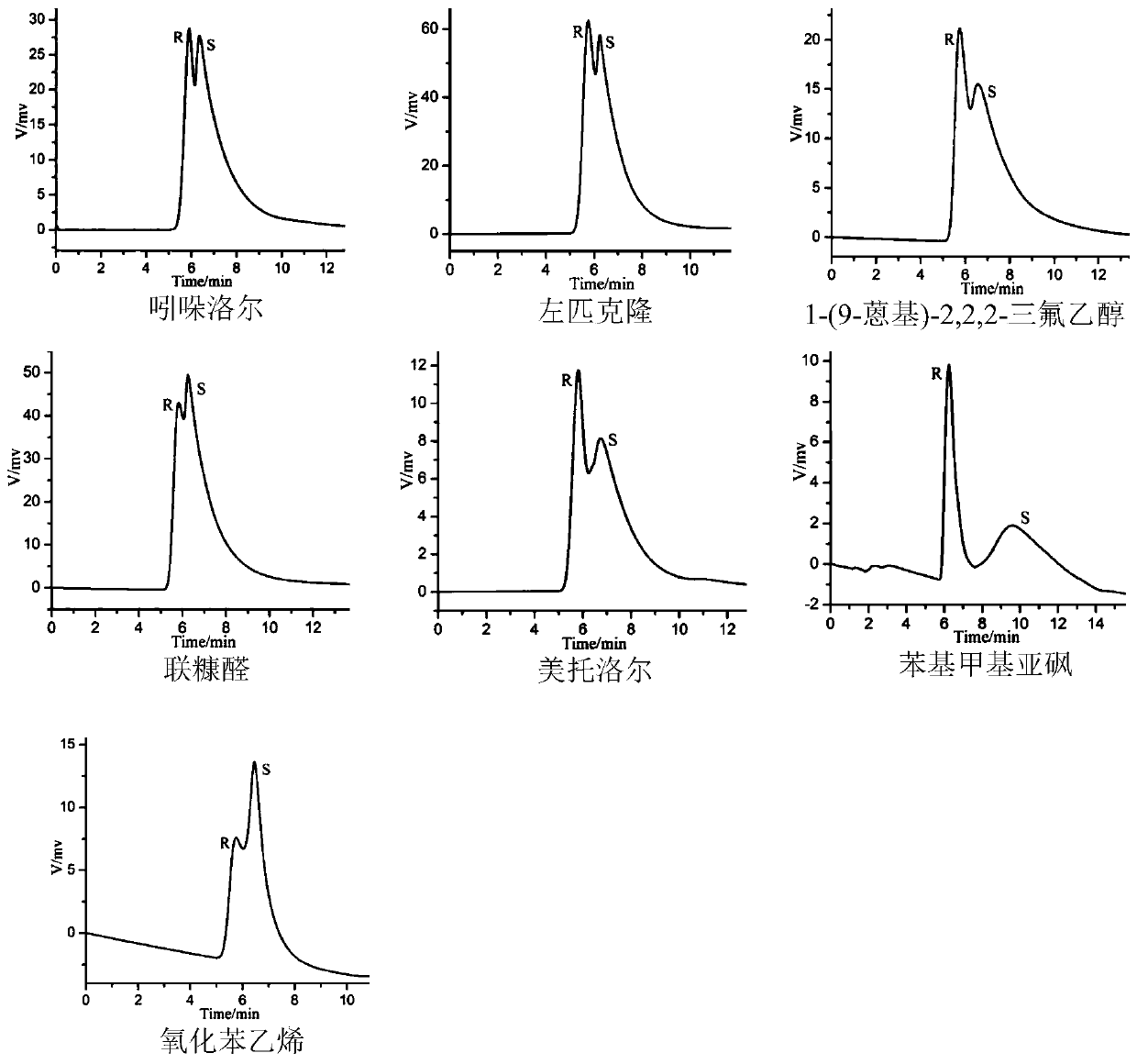
Image
Examples
Embodiment
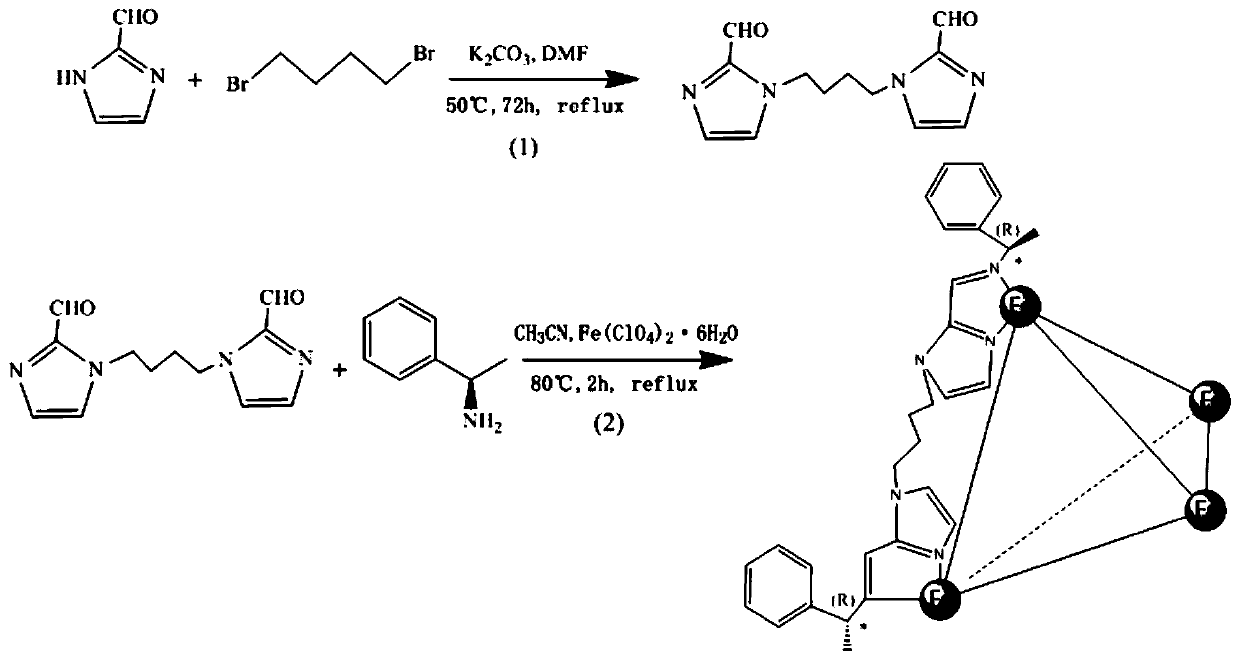
[0024] Synthesis of 1,4-bis(2-imidazole formaldehyde)butane: in N 2 Under protection, add 40mL of anhydrous DMF to a 100mL round-bottomed flask, then add 2-imidazole formaldehyde (2.6906g), 1,4-dibromobutane (2.1594g) and potassium carbonate (2.7642g) successively, at 50°C , the reactant was stirred for 72 hours, the residue was removed by filtration, and the filtrate was washed with CH 3 COOEt was extracted 4 times, the collected organic phase layer was washed 3 times with saturated potassium chloride solution, the organic phase was collected and dried with anhydrous magnesium sulfate, the solid-liquid was separated to obtain the filtrate, which was rotary evaporated at 40 °C and dried in vacuo to obtain the desired yellow needle. shape crystal;
[0025] Chiral metal-organic cages [Fe 4 L 6 ](ClO 4 ) 8 Synthesis of Solvent: 1,4-bis(2-imidazole formaldehyde)butane (98.4mg), (R)-1-phenylethylamine (97.8mg) and Fe(ClO 4 ) 2 ·6H 2 O (96.8 mg) was added to 20 mL of acetoni...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com