Chiral titanium complex as well as preparation and application thereof
A complex and chiral technology, applied in the preparation of carboxylate, analysis by nuclear magnetic resonance, organic chemistry, etc., can solve the problems of difficult synthesis, complex structure, disadvantages, etc., and achieve the effect of simple test operation and easy reagent synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
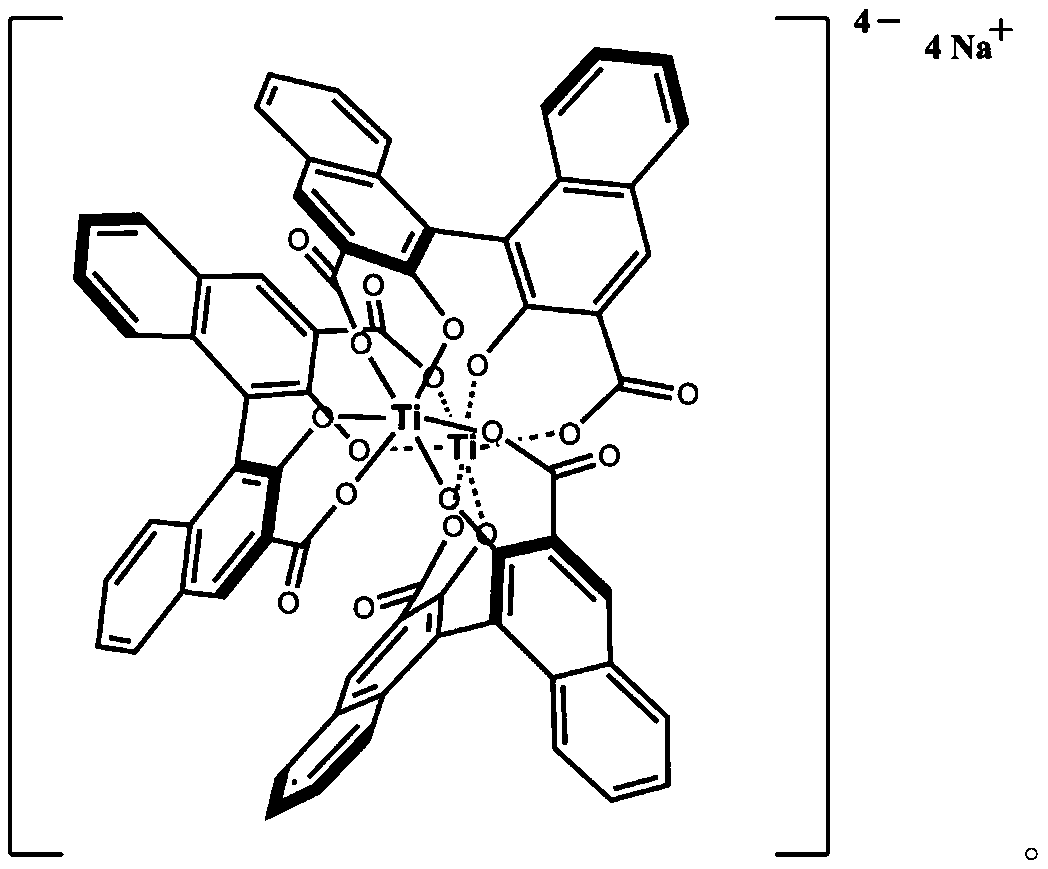
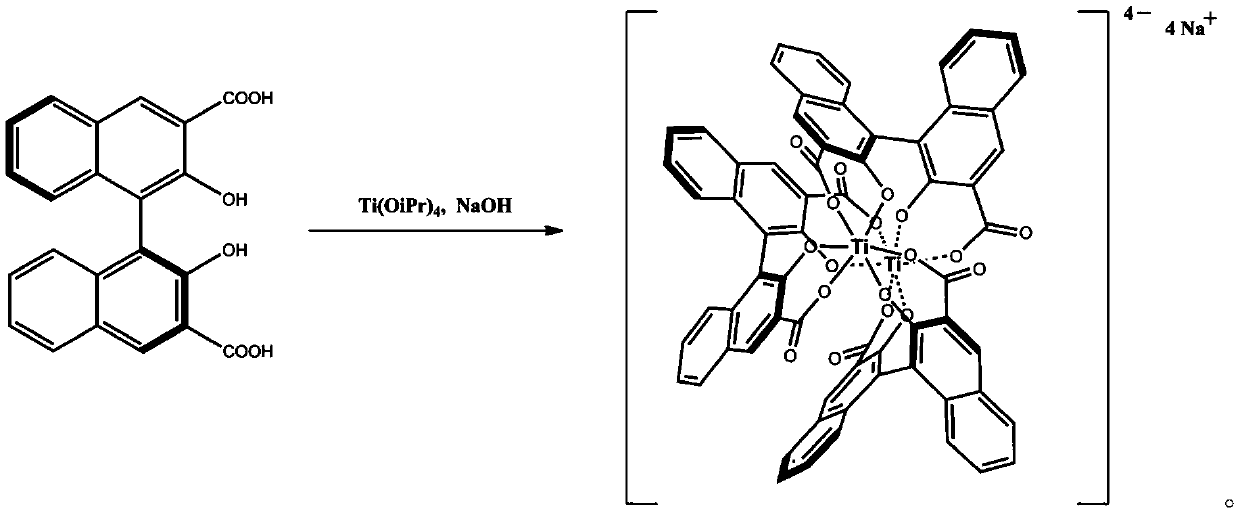
[0022] Synthesis of Example 1 Chiral Titanium Complex
[0023] Dissolve 1g of (S)-2,2'-dihydroxy-3,3'-binaphtalic acid in 40mL of methanol, add 0.5g of titanium isopropoxide dropwise at room temperature, continue to stir for 30min, and then add 0.14 g of sodium hydroxide, stir to completely dissolve, then heat up to 60°C, keep warm for 24 hours, then cool down to room temperature, concentrate the mixture under reduced pressure to remove the solvent, and recrystallize the residue with methanol and chloroform to obtain 0.81 g of the product as red crystals. The melting point is greater than 300°C. 1 H NMR (400MHz, CDCl 3 )δ7.63(s, 6H), 7.37(d, J=8.0Hz, 6H), 7.02(t, J=7.5Hz, 6H), 6.86(t, J=7.7Hz, 6H), 6.25(d, J = 8.6Hz, 6H); 13 C NMR (101MHz, CDCl 3 ) δ 169.54, 159.86, 136.22, 130.96, 129.86, 126.69, 125.74, 125.34, 125.02, 120.83, 116.04. Determination of the optical purity of the α-phenylethylamine sample of example 2 unknown optical purity
[0024] Weigh 1 mg of the α-ph...
example 3
[0025] Determination of optical purity of phenylglycine methyl ester hydrochloride sample of unknown optical purity in example 3
[0026] Weigh 1 mg of phenylglycine methyl ester hydrochloride sample to be tested and dissolve it in deuterated dimethyl sulfoxide, then add 6.6 mg of chiral titanium complex, mix well, put it into a 400 MHz nuclear magnetic resonance instrument and record at room temperature 1 H NMR signal, obtain the chemical shift and integral area of the split two groups of α-H peak signals, which is δ 1 =5.25,S 1 =0.365, δ 2 =5.19,S 2 = 0.635. (S 2 -S 1 ) / (S 1 +S 2 )=27.0% indicates that the optical purity of the sample to be tested is 27.0%.
[0027] A brief list of other implementation cases is as follows:
[0028]
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com