Preparing method of (2R,4R)-4-methylpiperidine-2-ethyl formate compound
A technology of ethyl piperidinecarboxylate and ethyl piperidinecarboxylate hydrochloride, which is applied in the field of preparation of ethyl-4-methylpiperidine-2-carboxylate, and can solve unfavorable large-scale production and complicated operation of isomers , Catalysts are expensive and other problems, to achieve the effect of easy operation during scale-up, simple recrystallization method, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
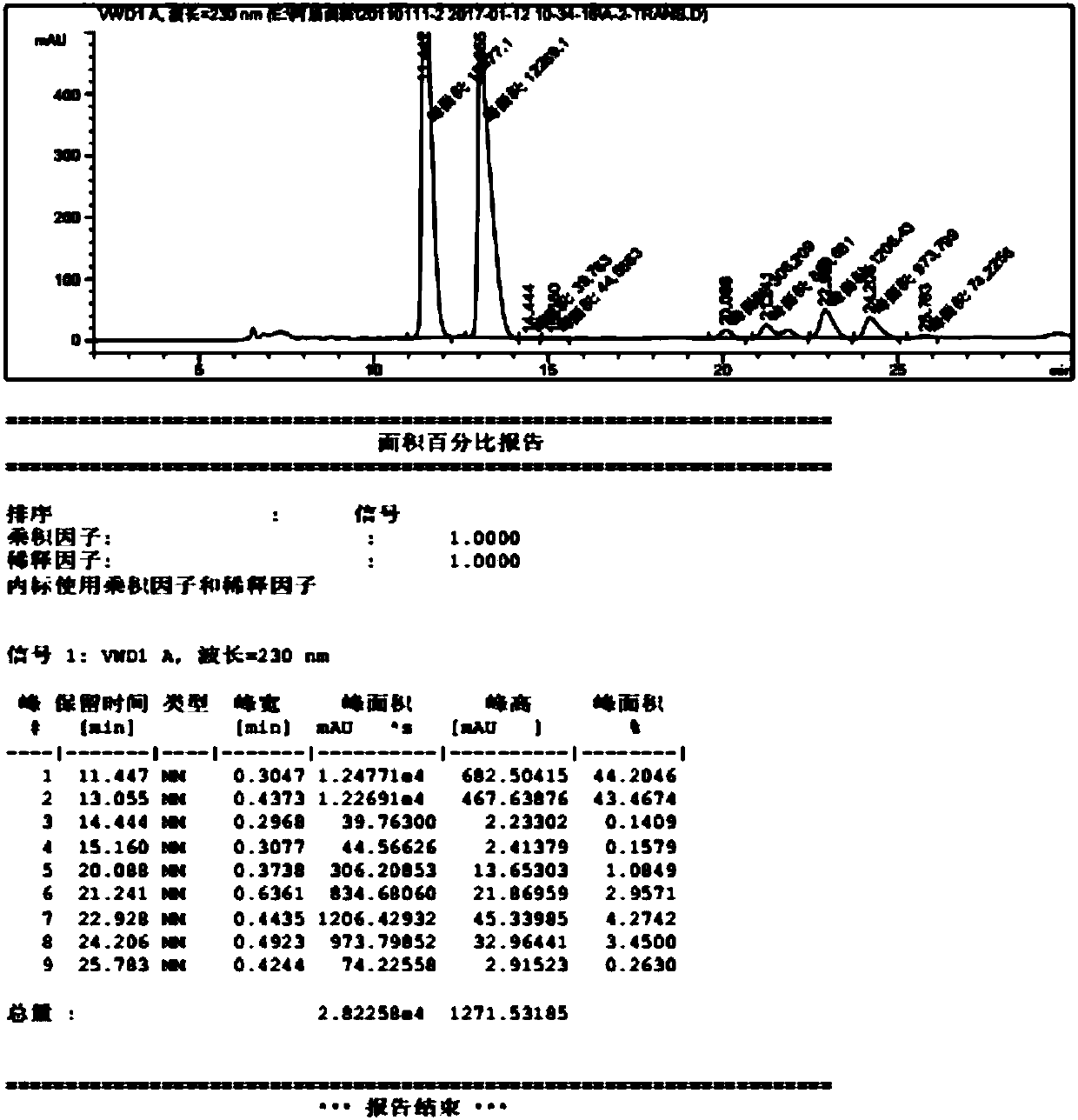
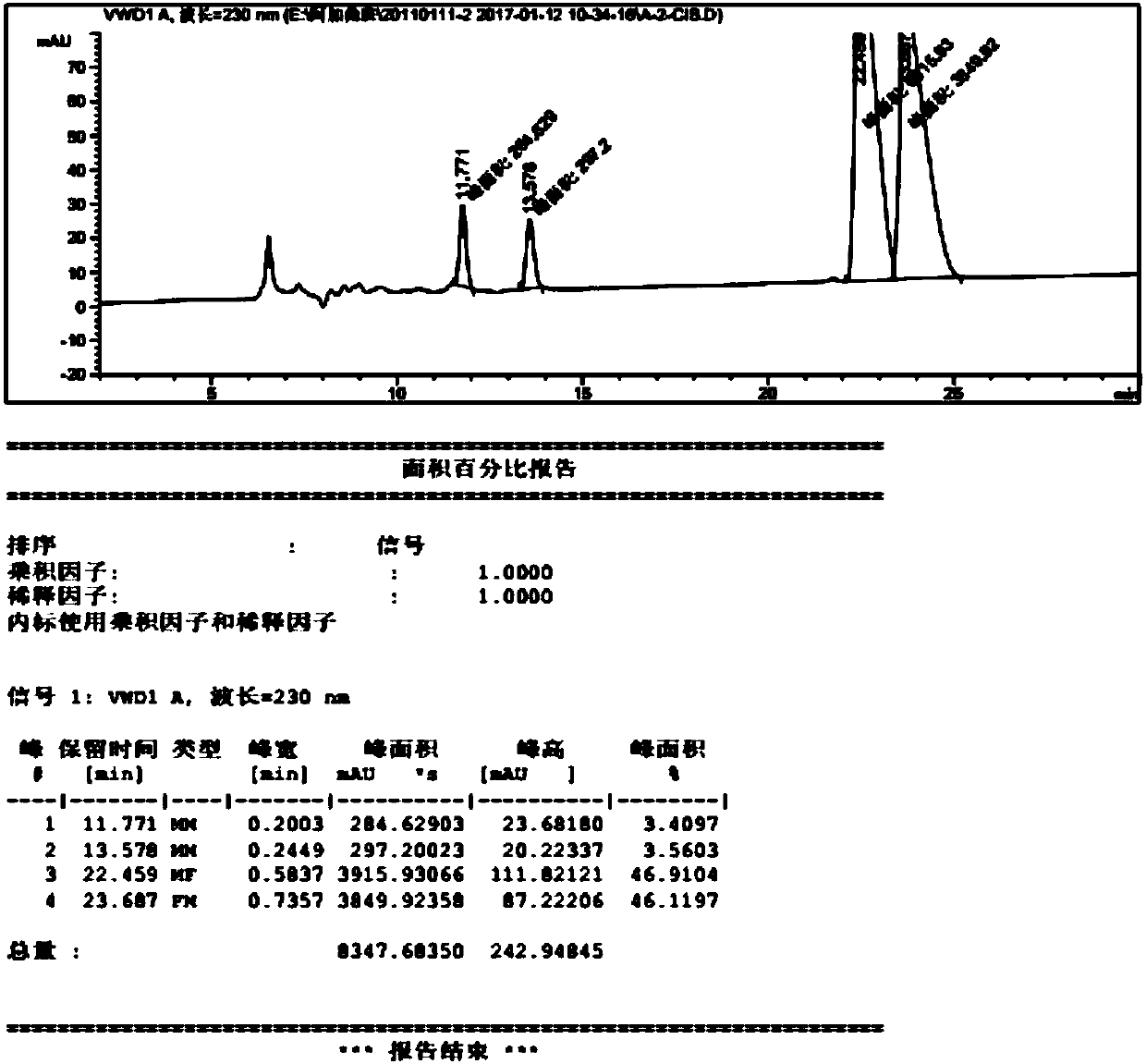
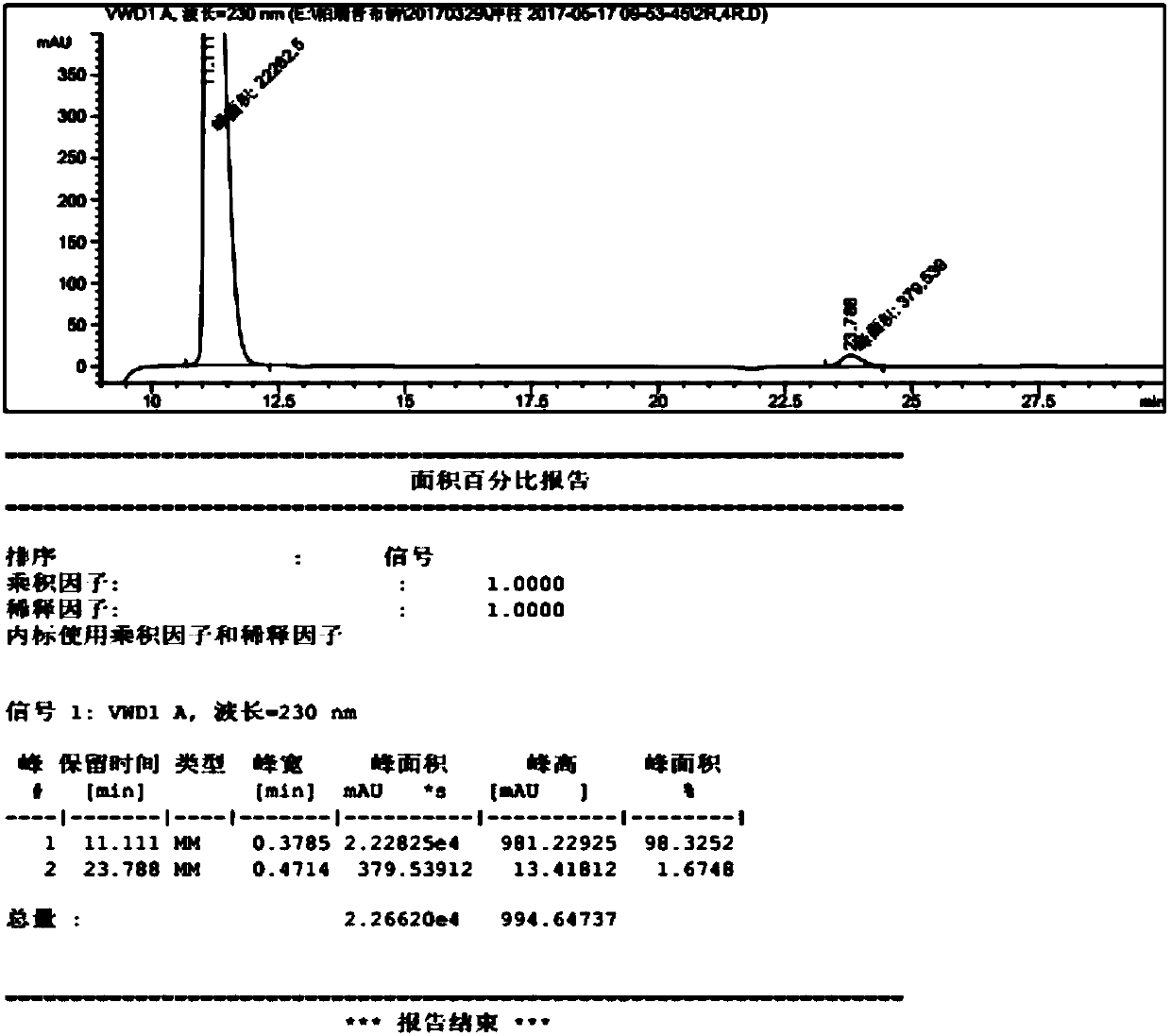
Image
Examples
Embodiment 1
[0052] 1. Preparation of 4-methylpiperidine-2-carboxylate hydrochloride
[0053] Add 16.0L of 6N hydrochloric acid and 2.0kg of 4-methyl-2-cyanopiperidine to a 50L glass reaction kettle, heat up to 100°C ± 5°C, and reflux for 5h. After the reaction, the system is evaporated under reduced pressure. After the solvent is evaporated, add 20.0L of absolute ethanol to the reaction kettle, reflux and stir for 1 h, drop to room temperature, filter, remove inorganic salts, collect the mother liquor and evaporate it to dryness under reduced pressure to obtain a solid, add 1.5L of absolute ethanol, 15.0L of methyl alcohol 2.1kg of 4-methylpiperidine-2-carboxylate hydrochloride, the molar yield of this step is about 73%.
[0054] Preparation of 4-methylpiperidine-2-carboxylic acid ethyl ester hydrochloride
[0055] In the glass reactor of 20L, add the absolute ethanol of 11.0L, add the 2.1kg of 4-methylpiperidine-2-formic acid hydrochloride obtained in the above steps into the reactor, s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| crystallization temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com