Polymorphic substance of triarylated dimethylpiperazine di-hydrochloride and preparation method and application thereof
A technology of dimethylpiperazine and ketone dihydrochloride, applied in the field of medicine, can solve problems such as long onset time, inability to concentrate, sexual dysfunction, and confusion of thinking
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Example 1 (4-((R)-((2S,5R)-4-(3-fluorobenzyl)-(2,5-dimethylpiperazin-1-yl)(3-hydroxyphenyl) Preparation of polymorph B of methyl)phenyl)(4-methylpiperidin-1-yl)methanone dihydrochloride
[0077] Take 10g (4-((R)-((2S,5R)-4-(3-fluorobenzyl)-(2,5-dimethylpiperazin-1-yl)(3-hydroxyphenyl)methyl Base) phenyl) (4-methylpiperidin-1-yl) ketone into a glass bottle, add 200mL ethyl acetate to the glass bottle and stir evenly, then add 3.5mL concentrated hydrochloric acid with a volume percentage of 37.5%, at room temperature Stir, react for 3 hours, and dry in vacuo to obtain (4-((R)-((2S,5R)-4-(3-fluorobenzyl)-(2,5-dimethylpiperazin-1-yl ) (3-hydroxyphenyl)methyl)phenyl)(4-methylpiperidin-1-yl)methanone dihydrochloride polymorph B.
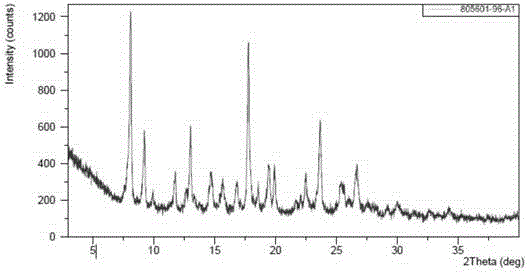
[0078] (4-((R)-((2S,5R)-4-(3-fluorobenzyl)-(2,5-dimethylpiperazin-1-yl)(3-hydroxy The XRPD pattern of polymorph B of phenyl)methyl)phenyl)(4-methylpiperidin-1-yl)methanone dihydrochloride is shown in figure 1 The peak information of its spectrum...
Embodiment 2
[0082] Example 2 (4-((R)-((2S,5R)-4-(3-fluorobenzyl)-(2,5-dimethylpiperazin-1-yl)(3-hydroxyphenyl) Preparation of polymorph E of methyl)phenyl)(4-methylpiperidin-1-yl)methanone dihydrochloride
[0083] (4-((R)-((2S,5R)-4-(3-fluorobenzyl)-(2,5-dimethylpiperazin-1-yl)( 3-Hydroxyphenyl) methyl) phenyl) (4-methylpiperidin-1-yl) polymorph B of ketone dihydrochloride is used as a starting sample, placed in a glass bottle; the glass bottle Place it open in a large glass bottle filled with 100mL of acetonitrile or dichloromethane; seal the large glass bottle and place it at room temperature for 5 days, and take out the solid, which is polymorph E.
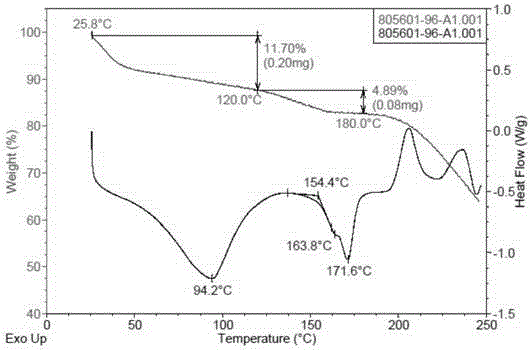
[0084] (4-((R)-((2S,5R)-4-(3-fluorobenzyl)-(2,5-dimethylpiperazin-1-yl)(3-hydroxy The XRPD pattern of polymorph E of phenyl)methyl)phenyl)(4-methylpiperidin-1-yl)methanone dihydrochloride is shown in image 3 The peak information of its spectrum is shown in Table 2. TGA and DSC diagrams of polymorph E Figure 4 shown by Figure 4 It ...
Embodiment 3
[0088] Example 3 (4-((R)-((2S,5R)-4-(3-fluorobenzyl)-(2,5-dimethylpiperazin-1-yl)(3-hydroxyphenyl) Preparation of polymorph H of methyl)phenyl)(4-methylpiperidin-1-yl)methanone dihydrochloride
[0089] (4-((R)-((2S,5R)-4-(3-fluorobenzyl)-(2,5-dimethylpiperazin-1-yl)( 3-Hydroxyphenyl) methyl) phenyl) (4-methylpiperidin-1-yl) ketone dihydrochloride polymorph B as the starting sample, add 100mL dimethyl sulfoxide to make the solid Completely dissolve; slowly add methyl isobutyl ketone dropwise until a precipitate is formed. Separate the precipitate, or transfer the sample to room temperature to quickly evaporate to obtain a solid, which is polymorph H.
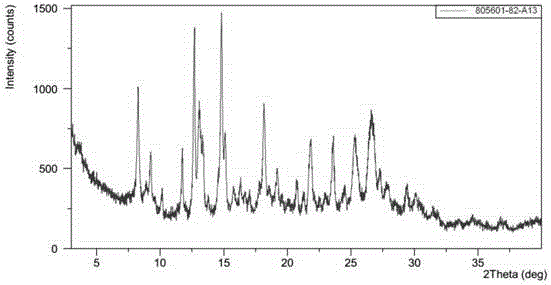
[0090] (4-((R)-((2S,5R)-4-(3-fluorobenzyl)-(2,5-dimethylpiperazin-1-yl)(3-hydroxy The XRPD pattern of polymorph H of phenyl)methyl)phenyl)(4-methylpiperidin-1-yl)methanone dihydrochloride is shown in Figure 5 As shown, the peak information of its spectrum is shown in Table 3. TGA and DSC diagrams of polymorph H Figure 6 sh...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com