Preparation method of tofacitinib citrate
A technology of tofacitinib and citric acid, applied in the field of chemical substances, can solve the problems of many side reactions, low yield, high cost of raw materials, etc., and achieve the effect of easy operation, high yield and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0025] The technical solutions in the present invention are clearly and completely described below in conjunction with the embodiments. Based on the embodiments of the present invention, all other embodiments obtained by persons of ordinary skill in the art without making creative efforts belong to the protection scope of the present invention.
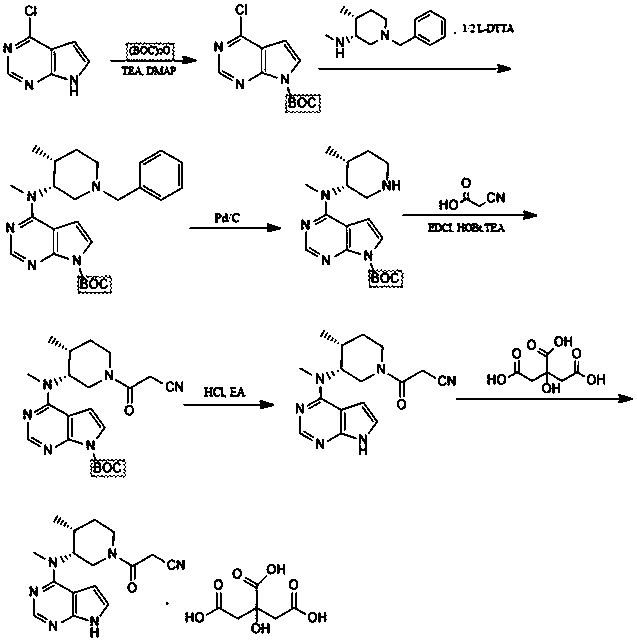
[0026] The present embodiment provides a kind of preparation method of tofacitinib citrate, such as figure 1 As shown, it specifically includes the following steps:
[0027] Step 1: Add 100ml of dichloromethane to the reaction flask, then add 10g of 4-chloro-7-pyrrolo[2,3-d]pyrimidine, then add 10g of triethylamine, then dropwise add 15g of (BOC) 2 O, stirred at room temperature, controlled reaction, after the reaction, concentrated the reaction solution, crystallized, filtered, washed with water, dried to obtain 18g of intermediate 1, the yield was 95%;
[0028] Step 2: Add 15g of intermediate 1 to 100ml DMF, add 25g of potassium c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com