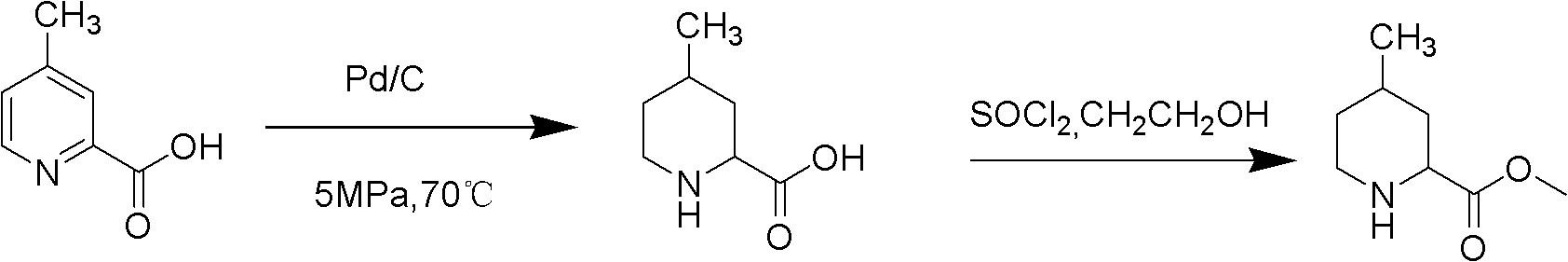
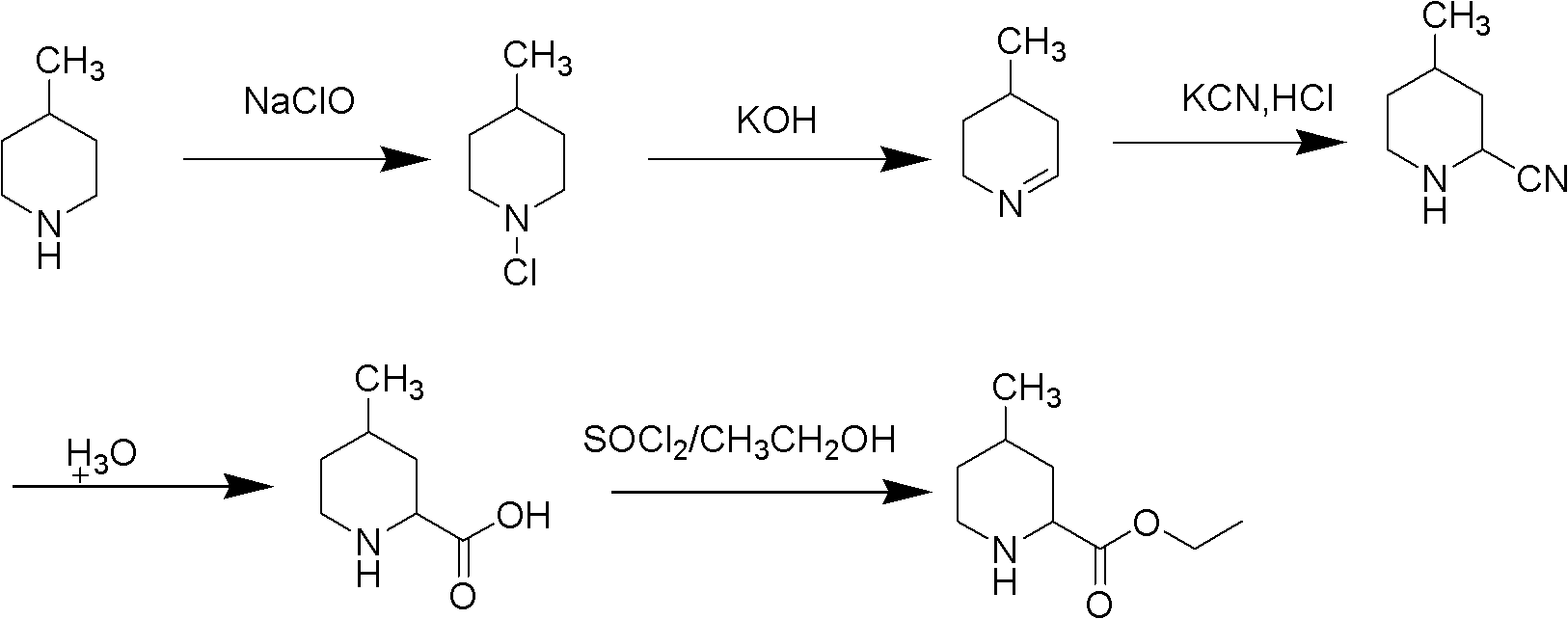
Method for preparing 4-methylpiperidine-2-carboxylate hydrochloride
A technology of ethyl carboxylate hydrochloride and methyl piperidine is applied in the field of preparation of ethyl 4-methylpiperidine-2-carboxylate hydrochloride, which can solve the complex cyclization synthesis route and the difficulty in product purification , industrialization difficulties and other problems, to achieve the effect of being conducive to industrial production, convenient purification, and excellent product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
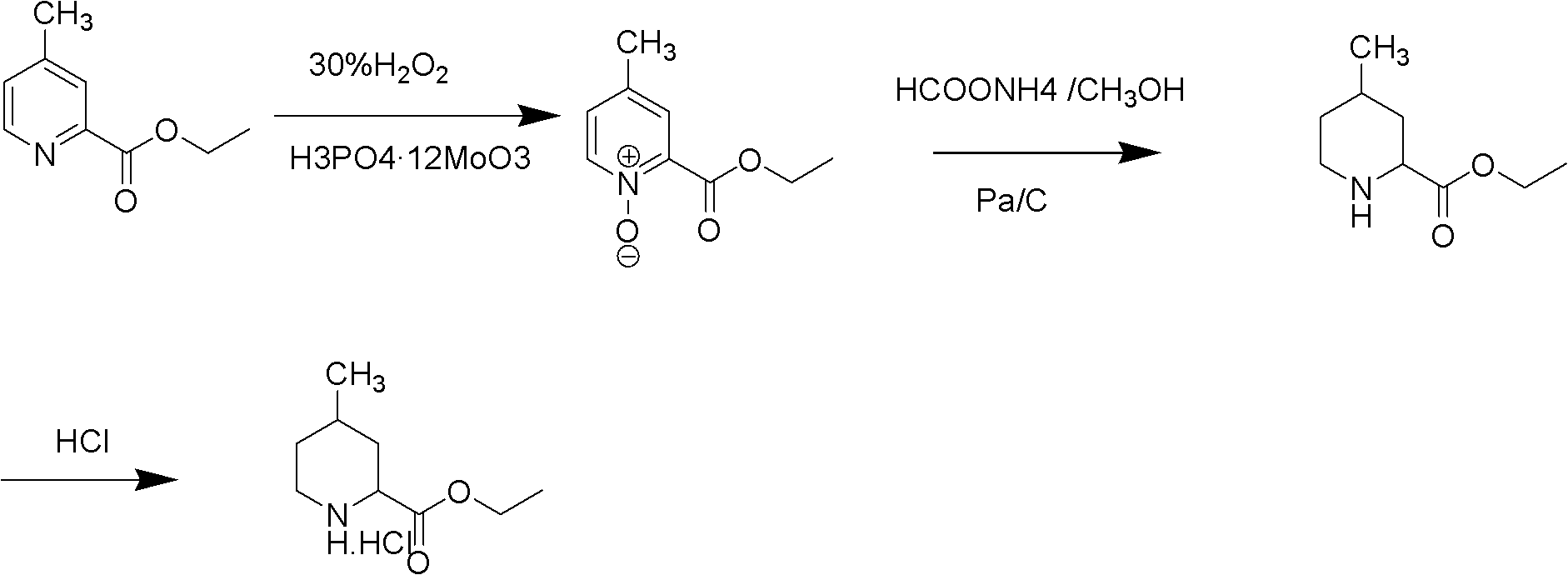
[0029] Preparation of ethyl 4-methylpyridine-2-carboxylate nitrogen oxide:
[0030] 50 grams (0.30 moles) of ethyl 4-picoline-2-carboxylate, 50 milliliters of purified water and 2.5 grams of phosphomolybdic acid are added in the reaction flask, and then 68 grams of 30% hydrogen peroxide are added dropwise, and the addition is completed. The temperature was raised to 70-80°C for reaction, followed by TLC until the reaction was complete. Cool the reaction solution to room temperature, adjust the pH value to about 9.0 with sodium carbonate, extract with 200 ml of dichloromethane*2, combine the organic layers, wash with 100 ml of purified water, separate the organic layer, dry with anhydrous magnesium sulfate, and concentrate To dryness, recrystallize from petroleum ether / ethyl acetate to obtain a light yellow solid with a yield of 92%.
[0031] Preparation of ethyl 4-methylpiperidine-2-carboxylate hydrochloride:
[0032] 50 grams (0.28 moles) of ethyl 4-picoline-2-carboxylate n...
Embodiment 2
[0034] Preparation of ethyl 4-methylpyridine-2-carboxylate nitrogen oxide:
[0035] 50 grams (0.30 moles) of ethyl 4-picoline-2-carboxylate, 50 milliliters of purified water and 2.5 grams of phosphomolybdic acid are added in the reaction flask, and then 68 grams of 30% hydrogen peroxide are added dropwise, and the addition is completed. The temperature was raised to 70-80°C for reaction, followed by TLC until the reaction was complete. Cool the reaction solution to room temperature, adjust the pH value to about 9.0 with sodium carbonate, extract with 200 ml of dichloromethane*2, combine the organic layers, wash with 100 ml of purified water, separate the organic layer, dry with anhydrous magnesium sulfate, and concentrate To dryness, recrystallize from petroleum ether / ethyl acetate to obtain a light yellow solid with a yield of 92%.
[0036] Preparation of ethyl 4-methylpiperidine-2-carboxylate hydrochloride:
[0037] 50 grams (0.28 moles) of ethyl 4-picoline-2-carboxylate n...
Embodiment 3
[0039] Preparation of ethyl 4-methylpyridine-2-carboxylate nitrogen oxide:
[0040] 50 grams (0.30 moles) of ethyl 4-picoline-2-carboxylate, 50 milliliters of purified water and 2.5 grams of phosphomolybdic acid were added to the reaction flask, and then 68 grams of 30% hydrogen peroxide was added dropwise, and the addition was completed. The temperature was raised to 70-80°C for reaction, followed by TLC until the reaction was complete. Cool the reaction solution to room temperature, adjust the pH value to about 9.0 with sodium carbonate, extract with 200 ml of dichloromethane*2, combine the organic layers, wash with 100 ml of purified water, separate the organic layer, dry with anhydrous magnesium sulfate, and concentrate To dryness, recrystallize from petroleum ether / ethyl acetate to obtain a light yellow solid with a yield of 92%.
[0041] Preparation of ethyl 4-methylpiperidine-2-carboxylate hydrochloride:
[0042] 50 grams (0.28 moles) of ethyl 4-picoline-2-carboxylate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com