Synthetic method of piperonal
A technology of jasmonaldehyde and synthesis method, applied in directions such as organic chemistry, can solve problems such as poor safety, many reaction steps, long routes, etc., and achieve the effects of high safety, few reaction steps, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
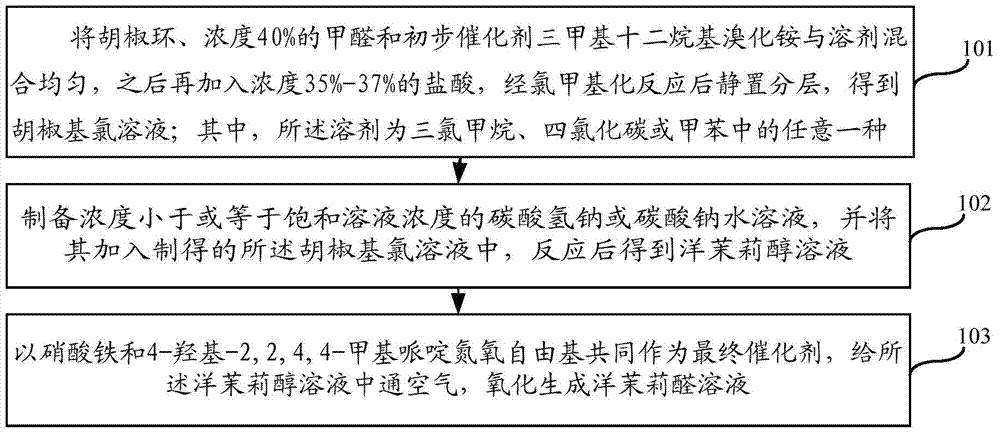
[0051] A kind of synthetic method of jasmonal, such as figure 1 shown, including the following steps:
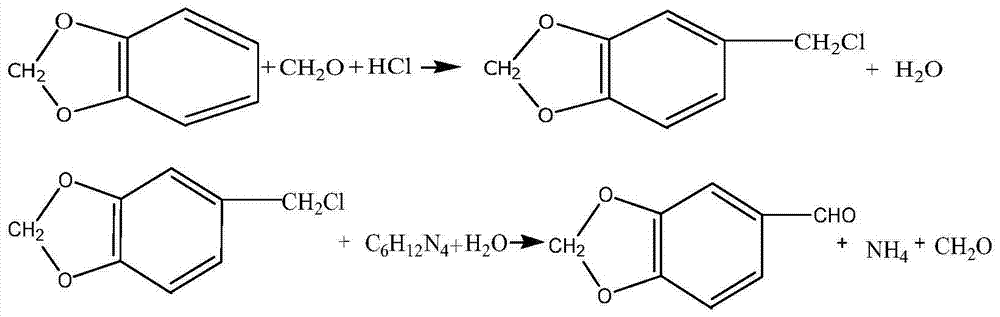
[0052]Step 1. Mix piperonyl cyclocycline, formaldehyde with a concentration of 40% and the preliminary catalyst trimethyldodecyl ammonium bromide with the solvent, then add hydrochloric acid with a concentration of 35%-37%, and statically Place the layers to obtain a piperonyl chloride solution; wherein, the solvent is any one of chloroform, carbon tetrachloride or toluene.
[0053] Prepare ingredients:
[0054] Measure piperonine, hydrochloric acid with a concentration of 35%-37%, and formaldehyde with a concentration of 40%, respectively, and the molar ratio of the three is 1:(1.2-1.5):(1.2-2.0);
[0055] In order to improve the utilization rate of raw materials, preferably, the molar ratio of piperonine, hydrochloric acid and formaldehyde is 1:(1.3-1.4):(1.4-1.6).
[0056] Measure the preliminary catalyst trimethyl dodecyl ammonium bromide, the amount of the preliminar...
Embodiment 1
[0085] In a 500mL flask, add 200ml of solvent chloroform, 61 grams (0.5mol) of piperonylcycline, 46.3 grams (0.63mol) of formaldehyde solution with a concentration of 40%, and 0.5 grams of catalyst trimethyldodecyl ammonium bromide, and stir evenly Finally, 60 grams (0.7 mol) of hydrochloric acid with a concentration of 36% was added dropwise, and the dropwise addition time was controlled at 150 minutes, and reacted at 60° C. for 12 hours, left still, and separated the lower layer oil to obtain a piperonyl chloride trichloromethane solution;
[0086] In the above piperonyl chlorotrichloromethane solution, slowly add unsaturated sodium carbonate solution while stirring, including 31.8 grams (0.3mol) of sodium carbonate, 200mL of water, stir and react at 60°C for 8 hours, and separate the lower organic phase It is jasmonol solution;
[0087] Add 200mL of water to the obtained jasminol solution, fully stir, and wash; two washes can be performed; stand and separate layers to obtai...
example 2
[0092] In 500mL flask, add solvent carbon tetrachloride 200ml, piperonyl cyclocyclone 61 grams (0.5mol), concentration be 46.3 grams (0.63mol) of formaldehyde solution of 40%, catalyzer trimethyl dodecyl ammonium bromide 0.5 grams, stir After being uniform, add 60 grams (0.7 mol) of hydrochloric acid with a concentration of 36% dropwise, and the dropwise addition time is controlled at 150 minutes, and react for 12 hours at 60° C., stand still, and separate the lower layer oil to obtain piperonyl chloride carbon tetrachloride solution;
[0093] In the above-mentioned piperonyl chloride carbon tetrachloride solution, slowly add unsaturated sodium bicarbonate solution while stirring, wherein 52 grams (0.6 mol) of sodium bicarbonate, 200 mL of water, stir and react at 60 ° C for 8 hours, separate The lower organic phase is a jasmonol solution;
[0094] Add 200mL of water to the obtained jasminol solution, fully stir, and wash; two washes can be performed; stand and separate layer...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com