Tofacitinib citrate impurity as well as analysis method and application thereof
A technology of tofacitinib and analysis method, which is applied in the field of the treatment of rheumatoid arthritis drug tofacitinib citrate impurities and its analysis, can solve problems such as harm and hidden dangers of drug safety, and achieve the goal of ensuring safe drug use and yield. The effect of high efficiency and simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1: Formula (I) compound 3-((3R,4R)-3-((7-hydroxyl-7H-pyrrolo[2,3-d]pyrimidin-4-yl)(methyl)amino)- Preparation of 4-methylpiperidin-1-yl)-3-oxopropionitrile
[0046] Weigh 3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)piperidin-1-yl)- Put 11.2g (30.35mmol) of 3-oxopropionitrile citrate and 9.9g (0.13mol) of peracetic acid into 358mL of methanol and 538mL of water, stir at 60-65°C for 22h, filter with suction, and concentrate the filtrate to remove methanol. Extract three times with dichloromethane, combine the organic phases, and wash with anhydrous Na 2 SO 4Dry, evaporate the solvent under reduced pressure, and purify by silica gel column chromatography, eluting with methanol:dichloromethane=1:15 to obtain 0.2g 3-((3R,4R)-3-((7-hydroxyl -7H-pyrrolo[2,3-d]pyrimidin-4-yl)(methyl)amino)-4-methylpiperidin-1-yl)-3-oxopropionitrile, yield 7.1%, The purity is 97.2%.
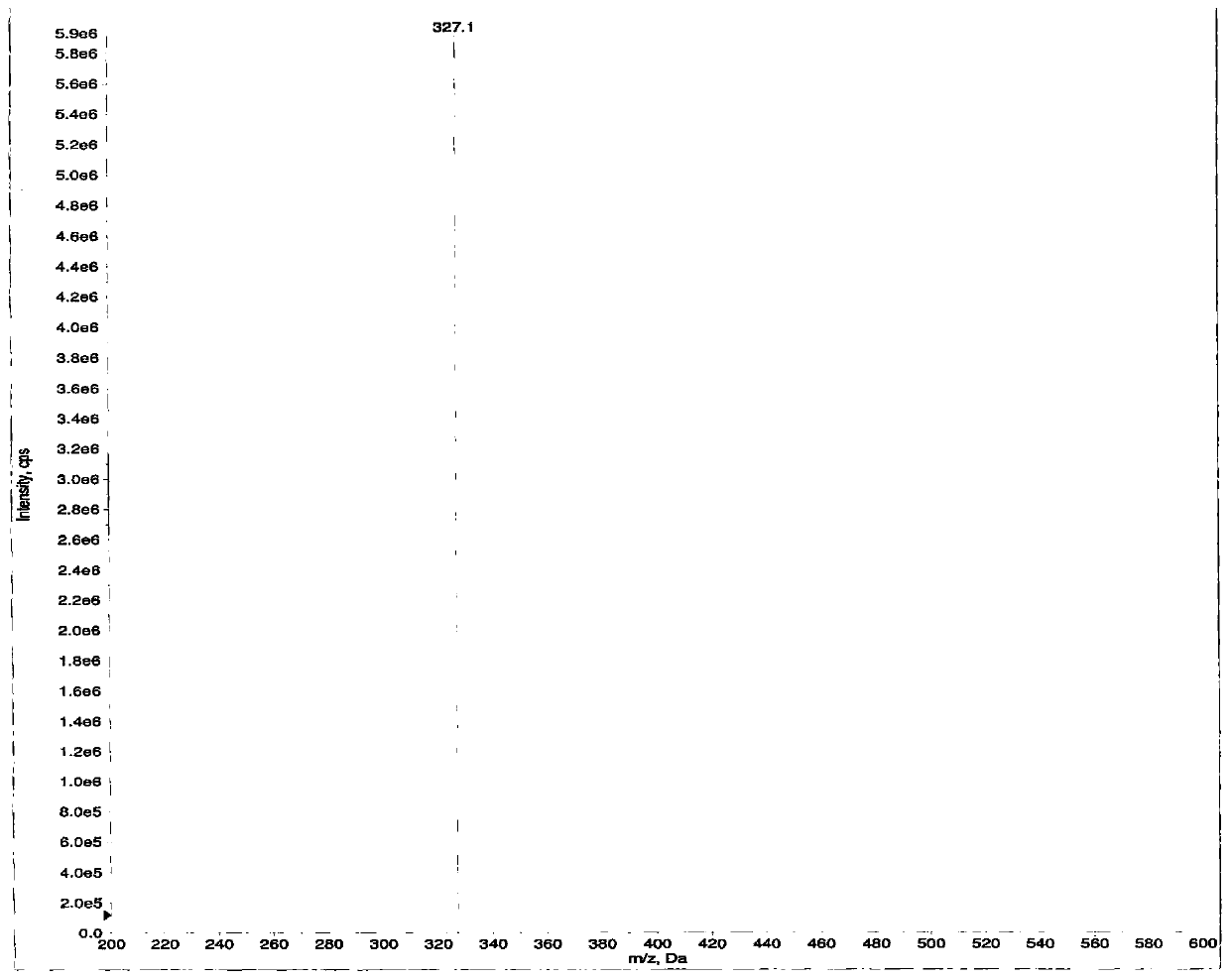
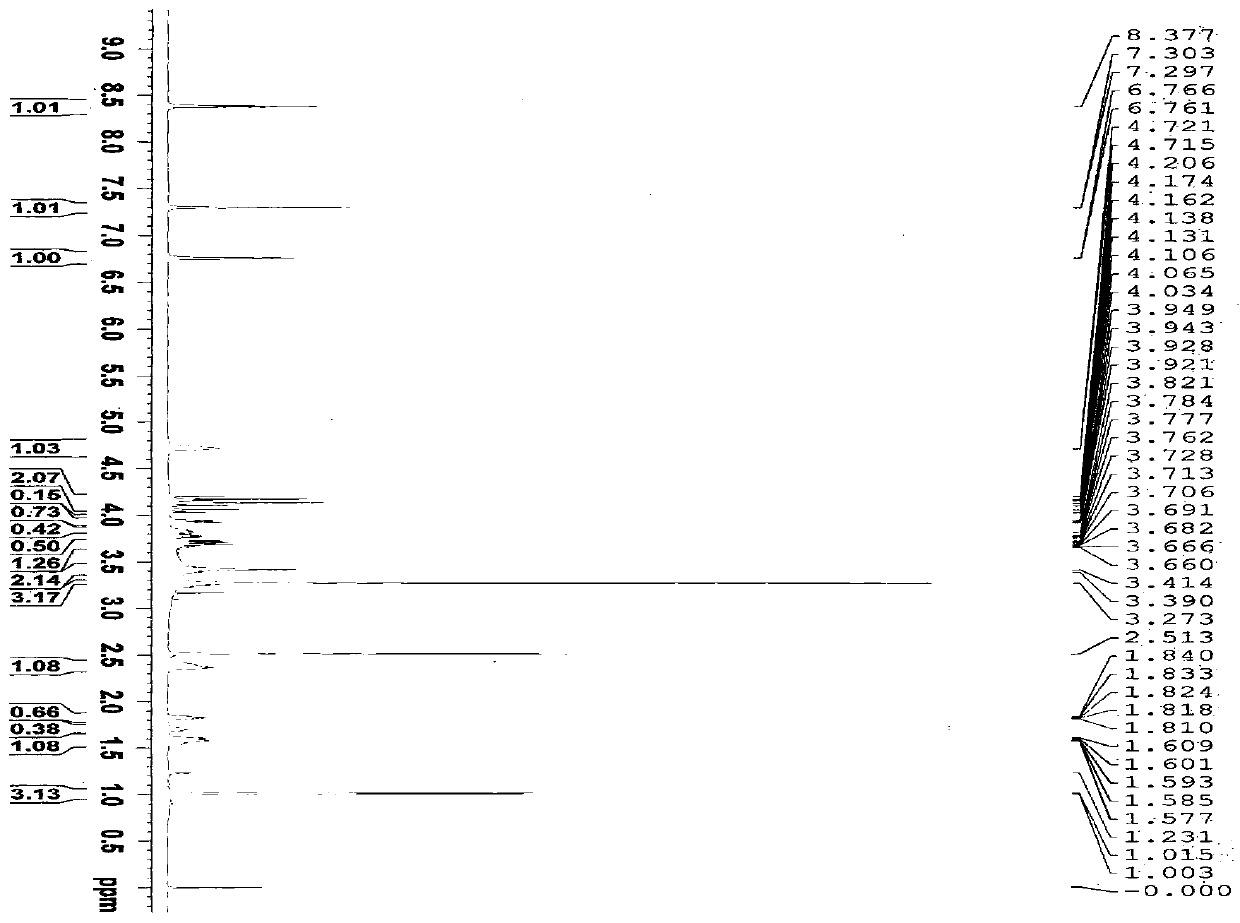
[0047] Confirm the structure of the prepared compound (I), the results are ...
Embodiment 2
[0051] Example 2: Formula (I) compound 3-((3R,4R)-3-((7-hydroxyl-7H-pyrrolo[2,3-d]pyrimidin-4-yl)(methyl)amino)- Preparation of 4-methylpiperidin-1-yl)-3-oxopropionitrile
[0052] Weigh 3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)piperidin-1-yl)- Add 11.2g (30.35mmol) of 3-oxopropionitrile citrate and 9.9g (0.13mol) of peracetic acid into 358mL of methanol and 538mL of water, stir at 50-55°C for 24h, filter with suction, and concentrate the filtrate to remove methanol. Extract three times with dichloromethane, combine the organic phases, and wash with anhydrous Na 2 SO 4 Dry, evaporate the solvent under reduced pressure, and purify by silica gel column chromatography, eluting with methanol:dichloromethane=1:15 to obtain 0.2g 3-((3R,4R)-3-((7-hydroxyl -7H-pyrrolo[2,3-d]pyrimidin-4-yl)(methyl)amino)-4-methylpiperidin-1-yl)-3-oxopropionitrile, yield 6.9%, The purity is 96.6%.
Embodiment 3
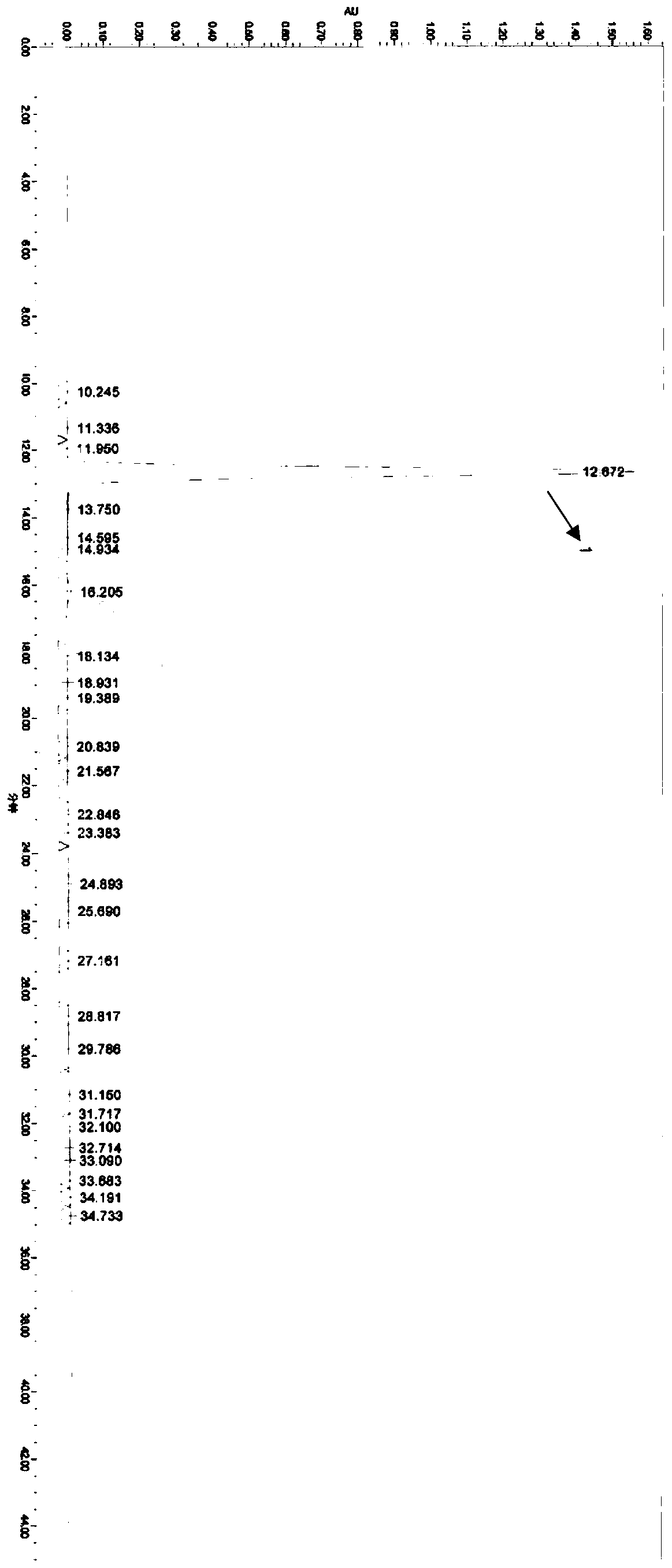
[0053] Example 3: 3-((3R,4R)-3-((7-hydroxyl-7H-pyrrolo[2,3-d]pyrimidin-4-yl)(methyl) in tofacitinib citrate Content determination of amino)-4-methylpiperidin-1-yl)-3-oxopropionitrile
[0054] Chromatographic conditions:
[0055] Column: CAPCELL PAK MGⅡC 18 Column, length 250mm, inner diameter 4.6mm, packing particle size 5μm;
[0056] Column temperature: 35°C;
[0057] Flow rate: 1.0ml / min;
[0058] Detection wavelength: 217nm;
[0059] Injection volume: 20μl;
[0060] Diluent: 50% acetonitrile;
[0061] The mobile phase A is 10mmol / L ammonium dihydrogen phosphate buffer (adjusted to pH 6.5 with ammonia water), the mobile phase B is methanol, and the elution gradient is as follows:
[0062] time (min) Mobile phase A(%) Mobile phase B(%) 0 90 10 25 60 40 35 50 50 40 50 50 41 90 10 45 90 10
[0063] Test operation:
[0064] Preparation of formula (I) compound control solution: take 10 mg of this product, put it in a 5...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com