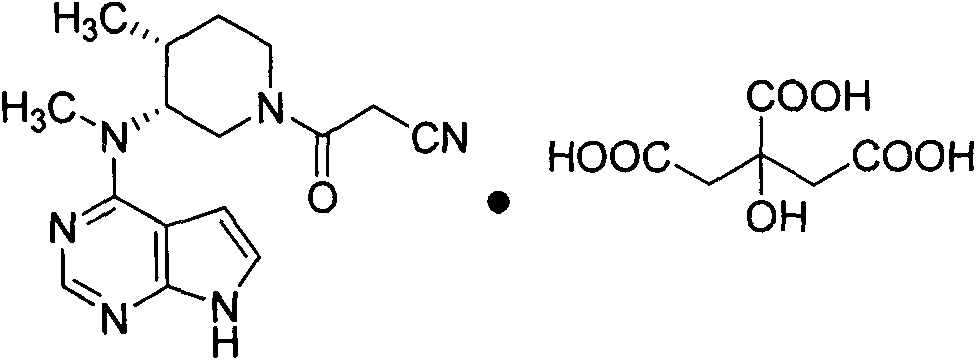
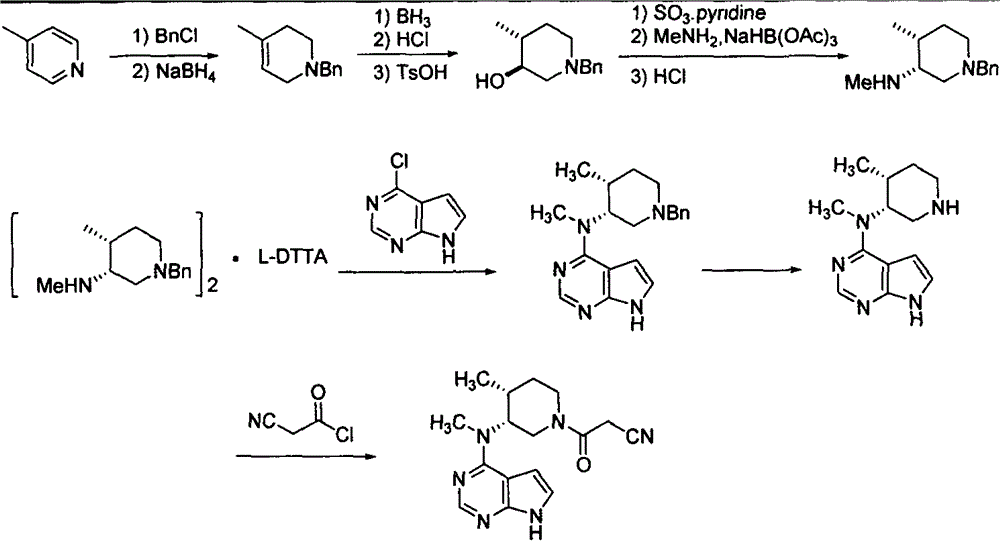
Synthesis method for tofacitinib
A methyl and pyrrole technology, applied in the field of synthesis of the JAK inhibitor tofacitinib, can solve the problems of large improvement space, short overall route, cumbersome operation, etc., and achieve the effect of simple operation, less impurities and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: the synthesis of cis-(4-methylpiperidin-3-yl) methyl carbamate
[0026]
[0027] Ethanol (150ml), (4-methylpyridin-3-yl) methyl carbamate (16.6g, 0.1mol), ethanol (150ml), concentrated sulfuric acid (10.9g, 0.11mol) and 5% Pd / C ( 3.5 g) were sequentially added to the hydrogenation kettle, replaced with nitrogen three times, replaced with 5MPa hydrogen three times, and reacted under 5MPa hydrogen pressure for 24h. Cool to room temperature, filter with suction, and concentrate the filtrate under reduced pressure to obtain a colorless oily liquid product (16.4 g, 95%). MS(m / z): 173[M+H] + ; 1 H NMR (300MHz, DMSO-d 6 ): 6.80 (d, J=8.7Hz, 1H, OCONH), 3.53 (s, 3H, CH 3 O), 3.47 ~ 3.49 (m, 1H, CHCH 2 NH), 2.70~2.81(m, 2H, CHCH 2 NH), 2.50~2.58(m, 2H, CHCH 2 CH 2 ), 2.40~2.42 (m, 1H, CHCH 2 NH), 1.68~1.73(m, 1H, CH 3 CH), 1.25~1.27(m, 2H, CHCH 2 CH 2 ), 0.78 (d, J=6.6Hz, 3H, CH 3 CH).
Embodiment 2
[0028] Example 2: Synthesis of cis-(1-benzyl-4-methylpiperidin-3-yl) methyl carbamate
[0029]
[0030] Add cis-(4-methylpiperidin-3-yl) methyl carbamate (85g, 0.49mol), DIPEA (95g, 0.74mol) and acetonitrile (400ml) into the reactor, keep the temperature below 10 degrees and drop Add benzyl chloride (60 g, 0.5 mol) in acetonitrile solution (200 ml) and let it rise to room temperature for 2 h. Evaporate the solvent under reduced pressure, add ice water (1L) to the residue, add dropwise 2mol / L hydrochloric acid (about 100ml) to adjust the pH to 3~4, extract with ethyl acetate, separate the layers, and use concentrated ammonia water (about 10ml) for the aqueous layer Adjust to pH 7-8, and then extract three times with dichloromethane. The organic layers were combined, dried over anhydrous sodium sulfate, and suction-filtered, and the filtrate was concentrated under reduced pressure to obtain a pale yellow oily product (110.3 g, 84%). MS(m / z): 263[M+H] + ; 1 H NMR (300MHz, ...
Embodiment 3
[0031] Embodiment 3, the synthesis of tofacitinib citrate
[0032]
[0033]N-[(3R,4R)-4-methylpiperidin-3-yl]-N-methyl-7H-pyrrolo[2,3-d]pyrimidin-4-amine (30g, 0.12mol), HOBT (23g, 0.17mol), cyanoacetic acid (13.6g, 0.16mol) and dichloromethane (300ml) were added to the reactor and reacted at room temperature for 12h. After the reaction was completed, add water (300ml) to quench, extract with dichloromethane, extract the aqueous layer with dichloromethane again, combine the organic layers, dry over anhydrous sodium sulfate and filter with suction, concentrate the filtrate under reduced pressure, add citrate monohydrate to the remaining light yellow solid A solution of citric acid (2.3g, 11mmol) in acetone (500ml) was stirred at 40°C for 2h. After cooling, stir overnight in an ice-water bath, filter with suction, wash the filter cake with acetone, and dry to obtain an off-white solid product (43 g, 70%). MS(m / z): 313[M+H] + ; 1 H NMR (500MHz, CD 3 OD): 11.62 (s, 1H, NH)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com