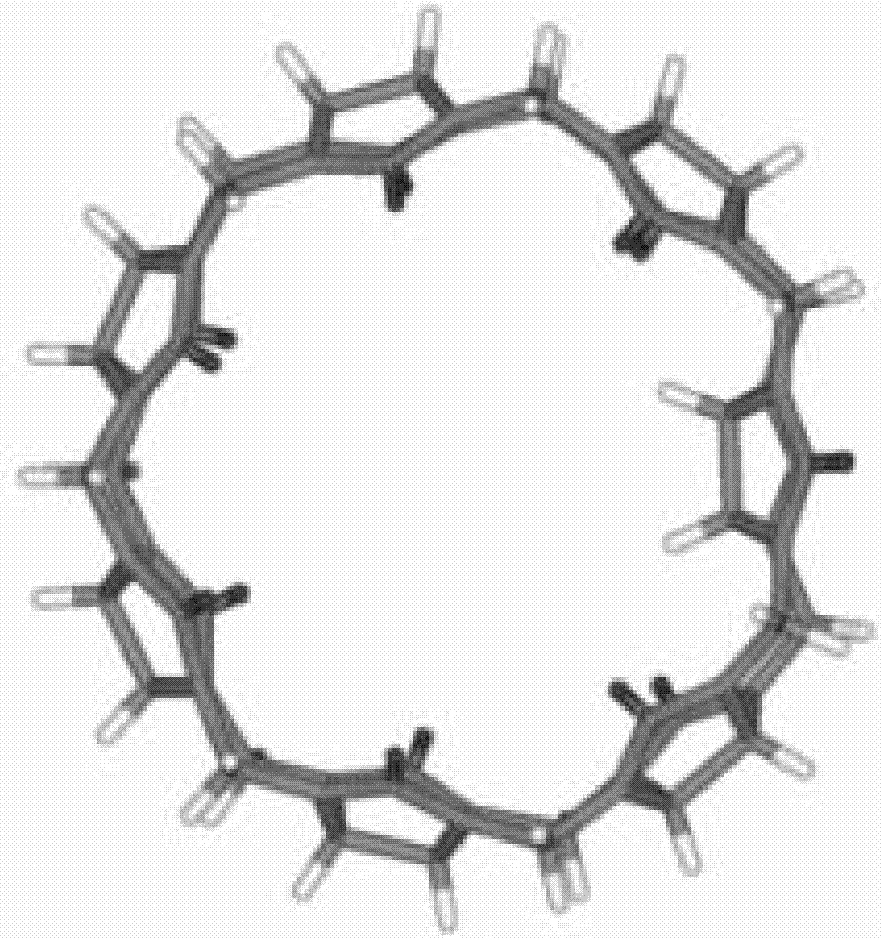
Application of trans-cucurbit(7)uril in recognition of biogenic amines
A seven-membered cucurbit ring, biogenic amine technology, applied in the field of supramolecular chemistry, can solve the problems of difficult separation, low content, restricting the development and application of trans cucurbit rings, etc., and achieves the effect of wide application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
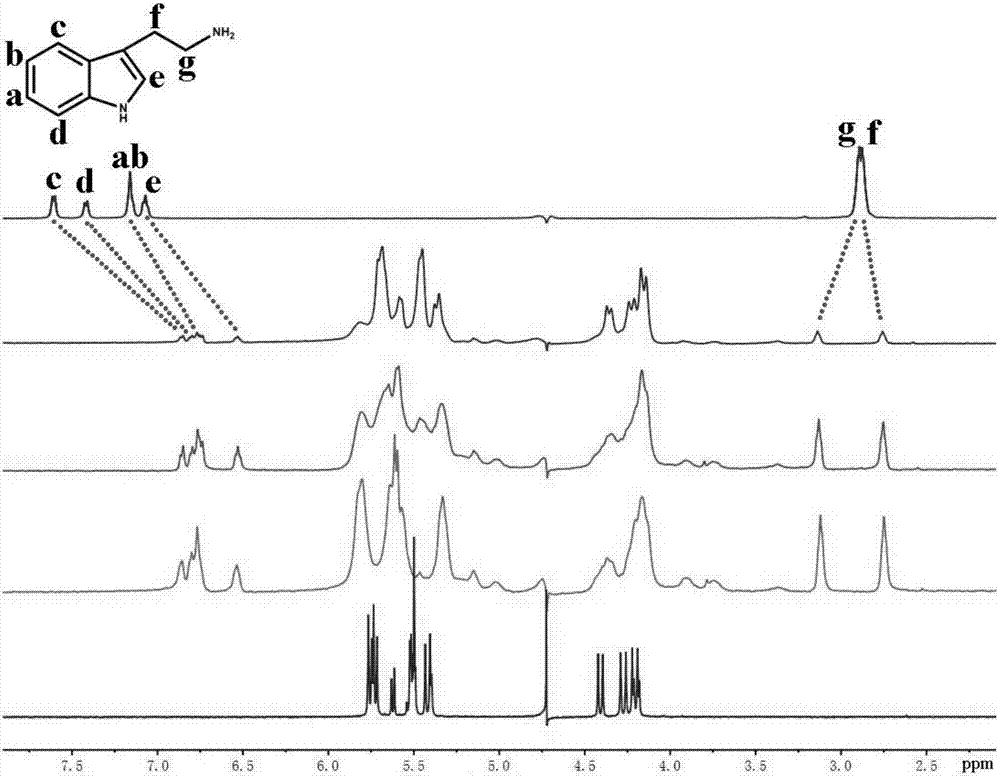
[0048] Example 1. A trans seven-membered cucurbit ring recognizes biogenic amines and is used to recognize tryptamine. The recognition method is as follows:
[0049] a. Put 2mg of trans seven-membered melon ring into the NMR tube, add 0.6mL of D 2 O shake, make it dissolve, get A product;
[0050] b. Put 2 mg of tryptamine in a cryovial, add 1.0 mL of D 2 O makes it dissolve to obtain product B;
[0051] c. Add product B to product A one by one, and get a corresponding NMR spectrum every drop. With the increase of tryptamine amount, a series of host-guest complexes are obtained (i.e., iQ[7] is the main body, Tryptamine as the guest host-guest complex) NMR spectrum, compare it with the tryptamine NMR spectrum, if there are two sets of signal peaks Hg and Hf in the spectrum, in which Hg moves to the lower field, and Hf moves to the higher field The field moves, and at the same time the signal peaks of the other Ha-He protons broaden and also move to the high field (such as ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com