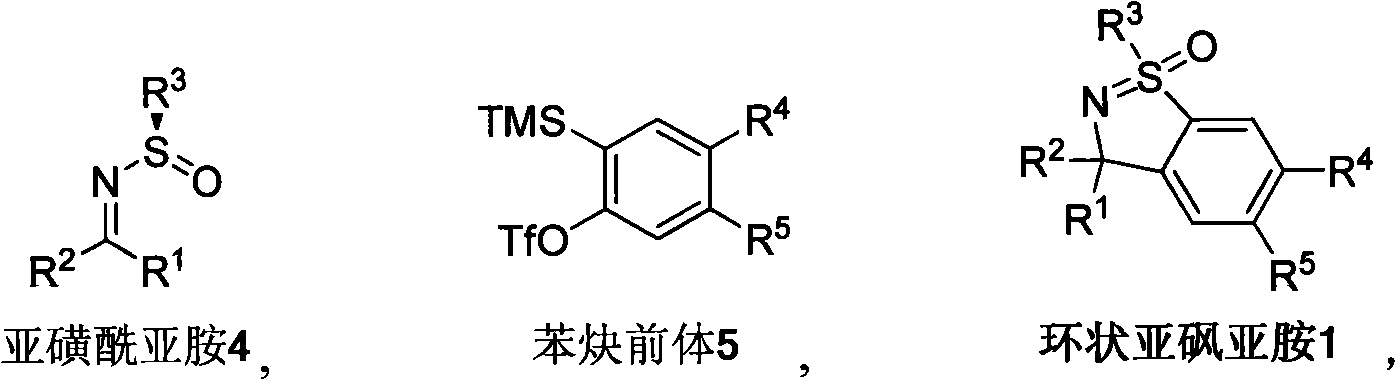
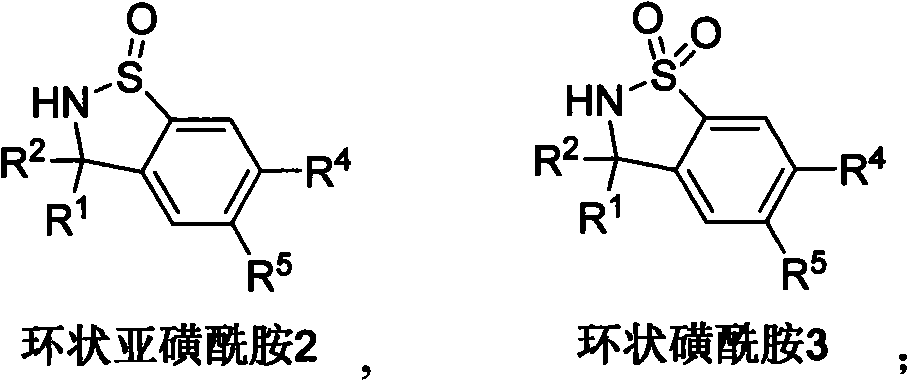
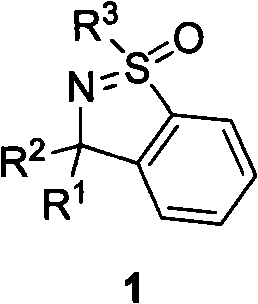
Method for synthesizing cyclic sulphoxide imine, sulfenamide and sulfamide by stereospecificty
A technology for sulfoximine and sulfenamide, which is applied in the field of efficient preparation of cyclic sulfoximine, can solve the problems of limited, unreported, and high cost of preparing cyclic sulfoximine, and achieves high stereospecificity, The effect of low cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Table 1
[0027]
[0028] Table 2
[0029]
[0030] Typical preparation method of cyclic sulfoximine 1:
[0031] At room temperature, to 4a (0.3mmol, 0.120g), 5a (0.9mmol, 0.268g), CH 3 CsF (1.5mmol, 0.228g) was added to the mixture of CN (5.0mL) and reacted at room temperature for 12h, followed by TLC to complete the reaction. Quenched with water, Et 2 O extraction (30mL×3), anhydrous MgSO 4 dry. The solvent was removed, and column chromatography (ethyl acetate:petroleum ether=1:3) gave 0.124g of product 1a with a yield of 87%.
[0032]
[0033] Mp: 138-140°C.[α] D 22 -45.1 (c 0.8, CHCl 3 ). 1 H NMR: δ8.03-7.87(m, 5H), 7.69-7.55(m, 3H), 7.47(t, J=7.4Hz, 3H), 7.26(d, J=7.4Hz, 3H), 1.47(s , 9H). 19 FNMR: δ-97.7 (d, J=233.9Hz, 1F), -100.0 (d, J=233.8Hz, 1F). 13 C NMR: δ 148.0 (d, J = 1.5Hz), 139.1, 136.2, 135.1, 134.8, 133.2, 131.3, 130.1, 128.9, 128.4, 128.2, 127.7 (d, J = 4.4Hz), 126.2 (d, J = 2.8 Hz), 123.8, 122.9 (t, J = 298.0 Hz), 63.8, 24.9 (d,...
Embodiment 2
[0087] table 3
[0088]
[0089] Typical preparation method of cyclic sulfoximine 1t:
[0090] At 0°C, Mg (4.0 mmol, 0.096g), the system naturally rose to room temperature, reacted for 8h, and TLC tracked the completion of the reaction. Add water, Et 2 O extraction (30mL×3), anhydrous MgSO 4 dry. The solvent was removed, and column chromatography (ethyl acetate:petroleum ether=1:3) yielded 0.063g of product 1t with a yield of 94%.
[0091]
[0092] [α] D 21 +20.2 (c 1.1, CHCl 3 ). 1 H NMR: δ7.83(d, J=7.8Hz, 1H), 7.80-7.70(q, J=7.4Hz, 3H), 7.63(t, J=7.4Hz, 1H), 7.55(t, J=7.5 Hz, 1H), 7.35-7.21(m, 3H), 6.15(t, J=55.9Hz, 1H), 1.61(s, 9H). 19 F NMR: δ-122.3 (dd, J=271.7, 56.9Hz, 1F), -123.4 (dd, J=271.7, 56.9Hz, 1F). 13 C NMR: δ147.7, 141.0, 136.9, 133.6, 130.6, 129.3, 128.7, 127.9(t, J=1.9Hz), 127.6(t, J=1.9Hz), 124.6, 118.2(t, J=250.7Hz) , 80.1 (t, J=21.8Hz), 63.9, 25.7. MS (ESI, m / z): 336.1 (M+H + ).HRMS(ESI): calcd.for C 18 h 19 f 2 NOS: (M+H + ): 336.12...
Embodiment 3
[0110] Table 4
[0111]
[0112] Typical preparation method of cyclic sulfinamide 2:
[0113] At -78°C, to 1a (0.2mmol, 0.084g), CH 2 Cl 2 (8mL) was slowly added to the mixture of pre-made HCl (Dioxane) (1.6mL, 2.5M) 【The HCl gas into the Dioxane, Dioxane as a carrier to absorb the HCl gas as a reactant, take Dioxane 1.6 containing 2.5M HCl gas mL], reacted at this temperature for 1h, and followed the completion of the reaction by TLC. Then add saturated NaHCO 3 Neutralize hydrochloric acid. CH 2 Cl 2 Extraction (30mL×3), anhydrous MgSO 4 dry. The solvent was removed, and column chromatography (ethyl acetate:petroleum ether=1:3) yielded 0.082g of product 2a with a yield of 98%.
[0114]
[0115] 1 H NMR: δ7.90(d, J=6.6Hz, 2H), 7.86-7.75(m, 3H), 7.69(t, J=7.3Hz, 2H), 7.60-7.46(m, 4H), 7.33(d , J=6.0Hz, 3H), 6.47(s, 1H). 19 F NMR: δ-99.3 (d, J=238.2Hz, 1F), -100.9 (d, J=238.1Hz, 1F). 13 C NMR: δ145.5, 137.9, 135.8, 135.4, 133.6, 131.9, 130.6, 130.4, 129.2, 129...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com