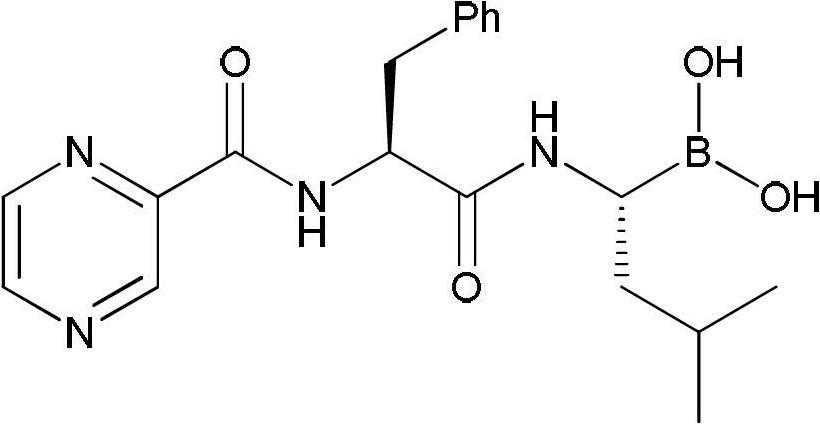
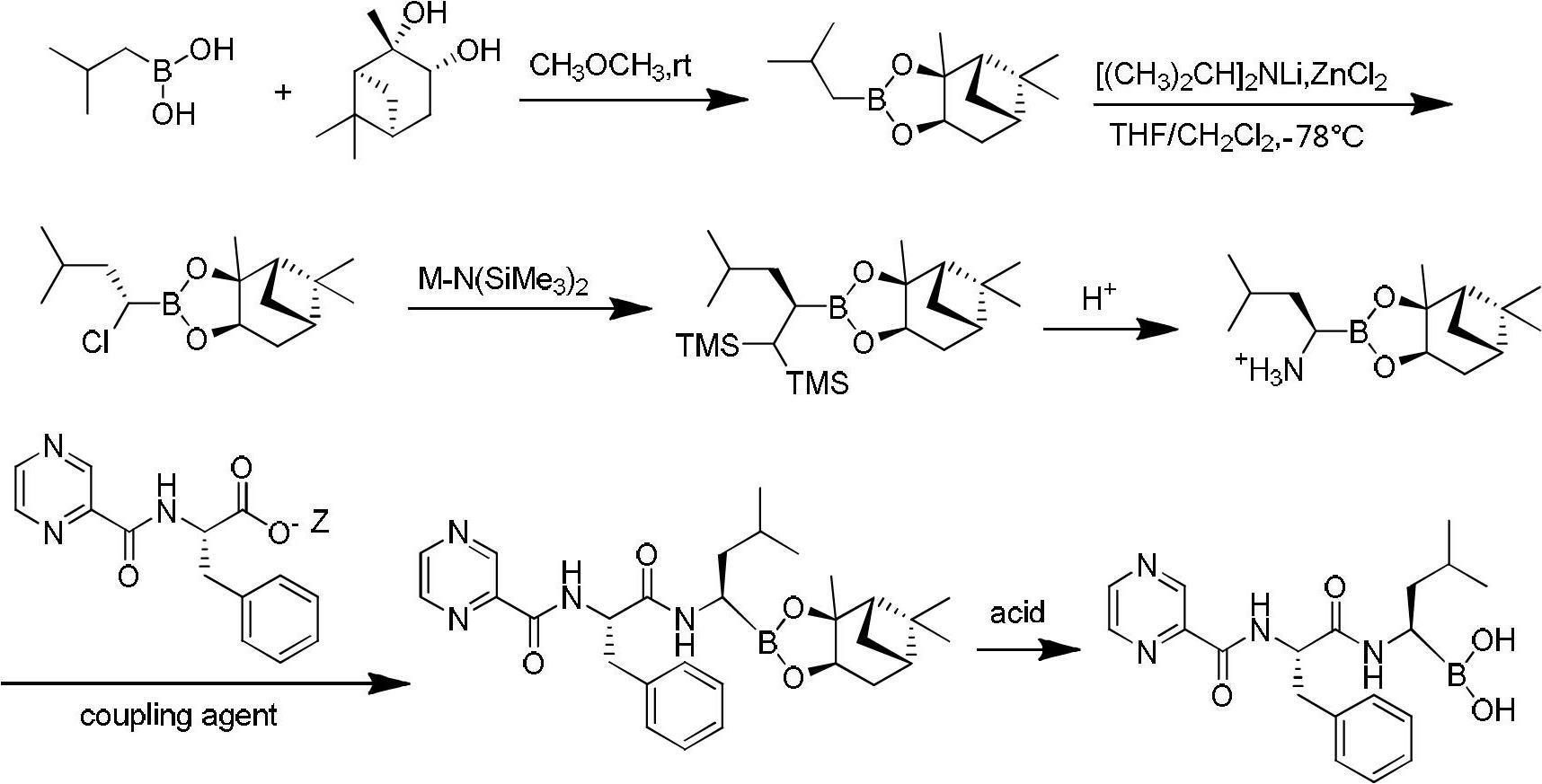
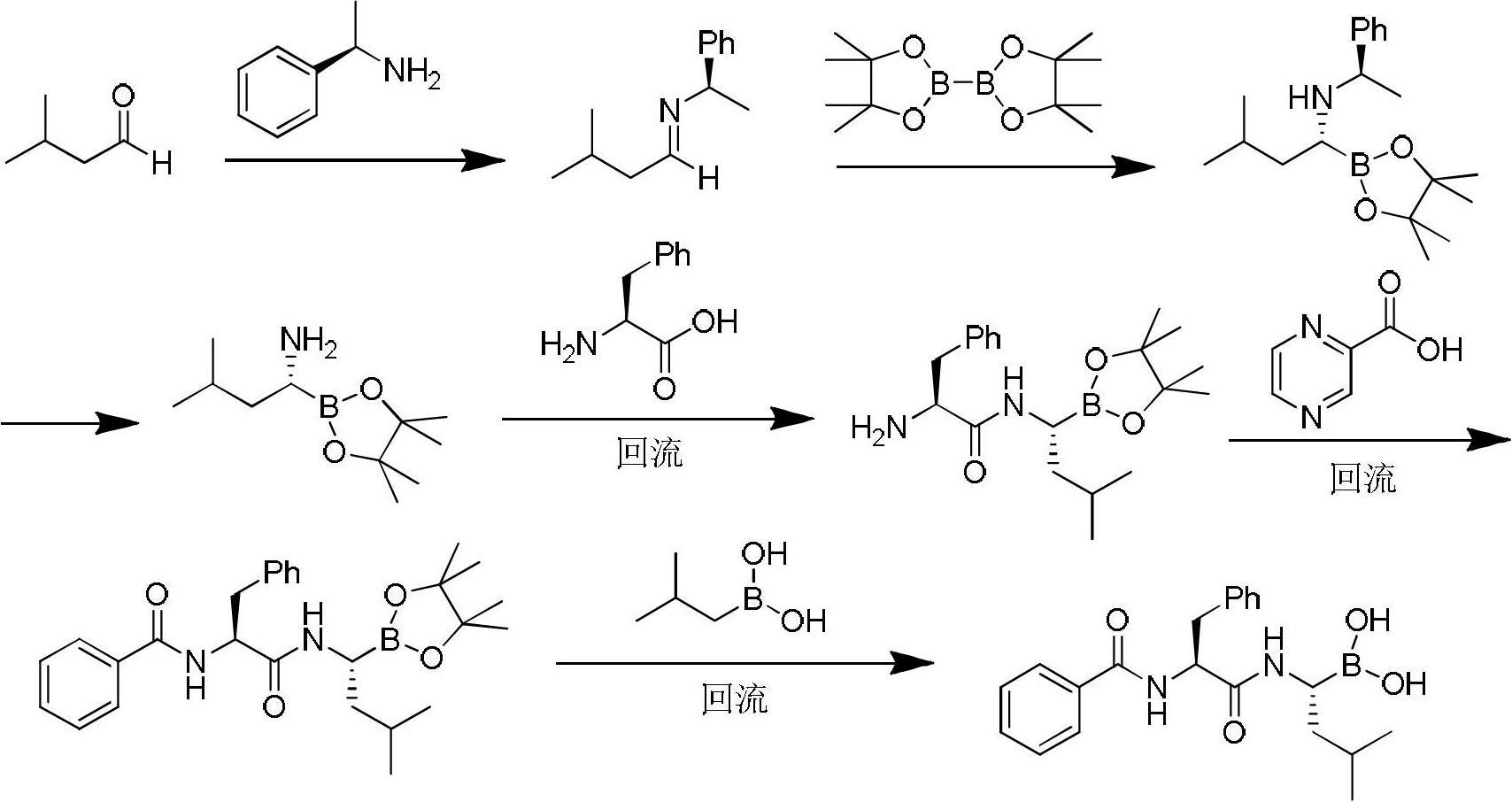
Synthetic method of bortezomib
A synthetic method, bortezomib technology, applied in the direction of peptides, etc., can solve the problems of inability to obtain compounds with a single configuration, expensive catalysts, low ee value, etc., to reduce the cost of synthetic raw materials, good quality of raw materials, and reaction The effect of mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: Preparation of (R,E)-2-methyl-N-(3-methylbutylene)propane-2-sulfinamide (Intermediate 1)
[0039] 1.1 Weigh 12.1g (100mmol) of (R)-tert-butylsulfinamide, 1.8g (15mmol) of magnesium sulfate and 0.37g (1.5mmol) of pyridinium 4-methylbenzenesulfonate into a 500mL round bottom flask, and then Take 13mL (120mmol) of isovaleraldehyde into the flask, and add 300mL of dichloromethane as a solvent, stir and react at room temperature for 23h, filter the reaction solution after the reaction, wash the filter residue three times with dichloromethane, keep the filtrate, and put it in a rotary evaporator The solvent was removed to obtain the crude product as a pale yellow liquid. The crude product was purified by column chromatography (eluent: petroleum ether: ethyl acetate = 5:1), and finally a colorless liquid was obtained which was Intermediate 1. The mass is 18.2g, and the yield is 95%.
[0040] 1.2 Weigh 12.1g (100mmol) of (R)-tert-butylsulfinamide into a 500mL round...
Embodiment 2
[0044] Example 2: Preparation of (R)-1-N-tert-butylsulfinyl-3-methylbutane-1-pinacol boronate (Intermediate 2)
[0045] 2.1 Under nitrogen protection, weigh 12.9g (50mmol) of pinacol diboron, 1.3g (5mmol) of 1,3-bicyclohexylimidazole hydrochloride, 0.5g (5mmol) of cuprous chloride and 0.96 sodium tert-butoxide g (10mmol) in a 250mL three-necked round-bottomed flask, add 18.8mL (50mmol) of the intermediate, and add 150mL of benzene as a solvent, stir at room temperature for 24 hours, add 100mL of ethyl acetate to the reaction solution after the reaction, filter, and keep Organic phase; the organic phase was washed with 100 mL of saturated sodium bicarbonate solution and separated, the aqueous phase was extracted with ethyl acetate and the organic phase was combined, dried and filtered with magnesium sulfate, concentrated, and separated by column chromatography with deactivated silica gel (washing Deliquified to petroleum ether: ethyl acetate = 10:1), spin-dried to obtain a colo...
Embodiment 3
[0050] Example 3: Preparation of pinacol-(R)-1-amino-3-methylbutane-1-boron ester hydrochloride (intermediate 3)
[0051] 3.1 Weigh 29.5g (30mmol) of the intermediate into a 250mL round bottom flask, add 150mL of 1,4-dioxane as a solvent to dissolve it completely, and then slowly add 4.0mol / L hydrogen chloride dioxane solution 7.5mL ( 30mmol), stirred at room temperature for 2h, and the solvent was removed by rotary evaporation to obtain a solid. The solid was washed three times with ether to obtain a white product which was Intermediate 3. The mass is 7.1 g, and the yield is 95%.
[0052] 3.2 Weigh 210.0g (31.5mmol) of the intermediate into a 250mL round bottom flask, add 150mL of 1,4-dioxane as a solvent to dissolve it completely, and then slowly add 4.0mol / L hydrogen chloride dioxane solution 7.8mL (31.5mmol), stirred at room temperature for 1.5h, and the solvent was removed by rotary evaporation to obtain a solid. The solid was washed three times with ether to obtain a wh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com