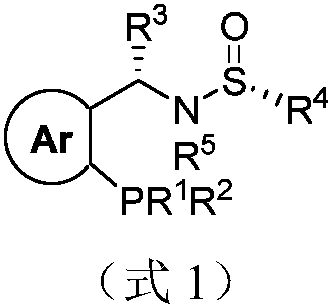
Sulfonamide type chiral monophosphine ligand as well as preparation method and application thereof
A sulfenamide and chiral technology, applied in the field of chiral monophosphine ligands and their preparation, can solve problems such as long synthetic routes, restrictions on the development of chiral phosphine ligands, and difficulty in transformation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
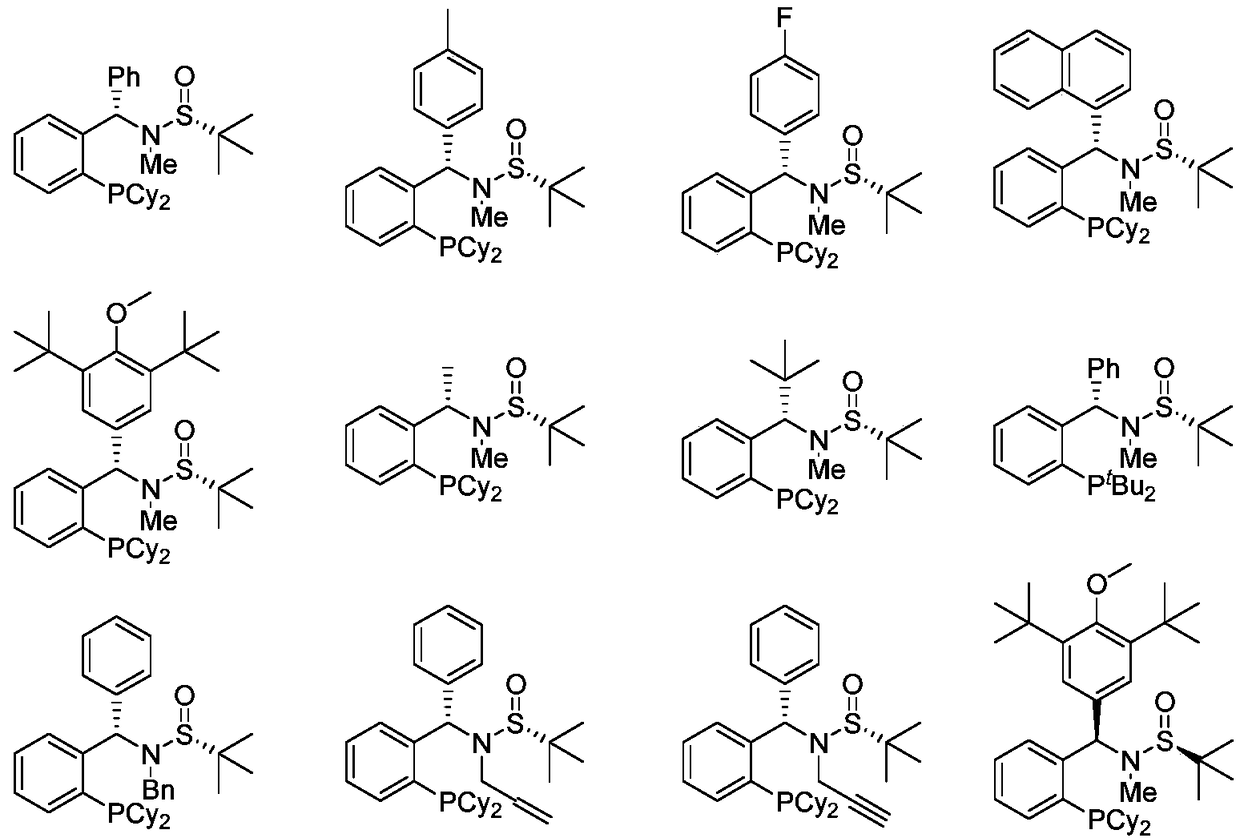
Examples
Embodiment 11a
[0045] Example 1 1a(S, R s )Synthesis
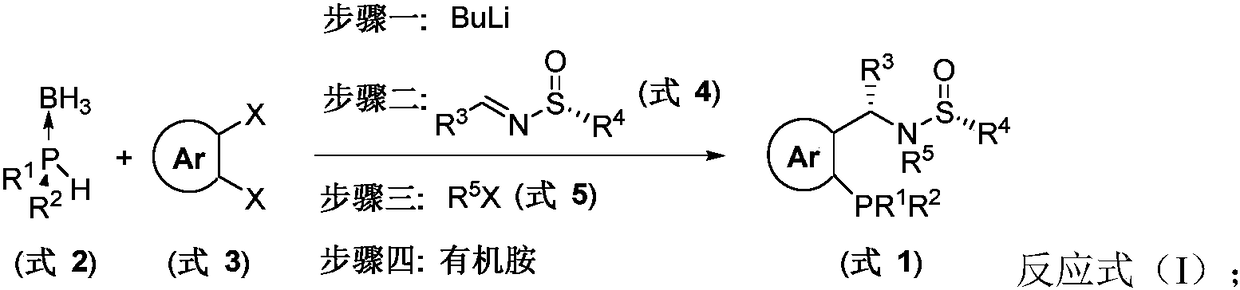
[0046] In a 250mL dry single-necked flask, add the borane complex of dicyclohexylphosphine (8mmol) and dry mixed solvent of toluene and tetrahydrofuran (50ml) under nitrogen atmosphere, and stir at -78°C for 10 minutes After that, n-butyllithium (1.0eq., 8mL, 1.6M) was added dropwise. Stirring was continued for 1.5 hours, and o-dibromobenzene (8mmol) was added, followed by n-butyllithium (8mmol, 1.6M). 30 minutes to join (9.6 mmol), then warmed to room temperature and stirred overnight. Thereafter, methyl trifluoromethanesulfonate (12 mmol) was added to the reaction system. Subsequently, select dry solvent, add Et 2 NH (8mL), stirred overnight at 50°C, spin-dried, and purified by column chromatography to obtain The yield was 50%. Proton NMR (300MHz, CDCl 3 )δ7.76-7.72 (m, 1H), 7.49-7.38 (m, 2H), 7.30-7.27 (m, 1H), 7.25-7.15 (m, 5H), 6.87 (d, J=10.1Hz, 1H) , 2.57(s, 3H), 1.91-1.41(m, 11H), 1.28-0.85(s, 20H). Phosphine NMR (122M...
Embodiment 21b
[0047] Example 2 1b(S, R s )Synthesis
[0048] Concrete operation with reference to embodiment 1, raw material used is The yield was 55%. Proton NMR (300MHz, CDCl 3 )δ7.73-7.69 (m, 1H), 7.48-7.37 (m, 2H), 7.28-7.23 (m, 1H), 7.07-7.01 (m, 4H), 6.82 (d, J=10.1Hz, 1H) , 2.56(s, 3H), 2.27(s, 3H), 1.91-0.87(m, 31H). Phosphine NMR (122MHz, CDCl 3 )δ-16.88. Carbon NMR (126MHz, CDCl 3 )δ147.37(d, J=22.2Hz), 137.02, 136.71, 134.93(d, J=21.1Hz), 132.95(d, J=3.2Hz), 130.41, 128.65, 128.57, 128.10(d, J=4.9 Hz), 126.43, 69.79(d, J=31.6Hz), 58.49, 34.67(dd, J=22.3, 13.1Hz), 30.63(d, J=18.0Hz), 29.98-29.34(m), 27.17-26.55( m), 26.26 (d, J=24.5Hz), 24.09, 21.00. High resolution mass spectrometry theoretical data C 30 h 45 NOPS: m / z (%): 512.3116 (M+H + ), experimental data: 512.3120.
Embodiment 31c
[0049] Example 3 1c(S, R s ) synthesis (reference scheme 1)
[0050] Concrete operation with reference to embodiment 1, raw material used is The yield was 43%. Proton NMR (300MHz, CDCl 3 ( s, 3H), 1.89-1.45 (m, 11H), 1.23-0.85 (m, 20H). Phosphine NMR (122MHz, CDCl 3 )δ-17.02. Fluorine NMR (282MHz, CDCl 3 )δ-115.16 (tt, J=8.1, 5.0Hz). Carbon NMR (126MHz, CDCl 3 )δ161.86 (d, J=246.5Hz), 146.90 (d, J=22.1Hz), 135.84 (d, J=3.3Hz), 134.84 (d, J=21.4Hz), 133.22 (d, J=3.3 Hz), 132.37(d, J=8.1Hz), 128.92, 127.76(d, J=4.8Hz), 126.73, 114.70(d, J=21.3Hz), 69.58(d, J=32.2Hz), 58.62, 34.69 high Resolution Mass Spectrometry Data C 30 h 45 NOPS: m / z (%): 516.2865 (M+H + ), experimental data: 516.2860.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com