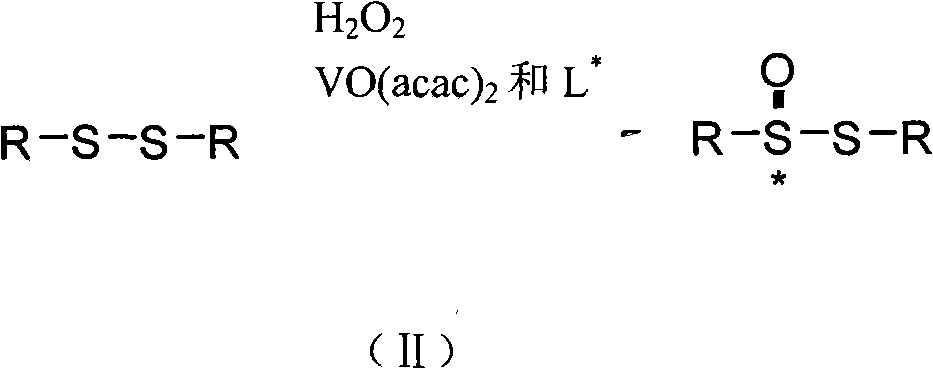
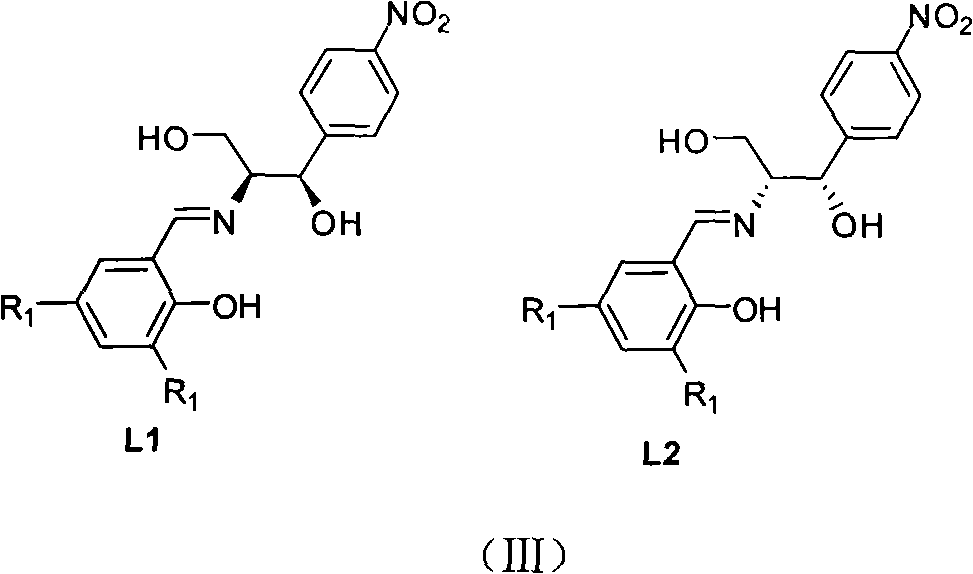
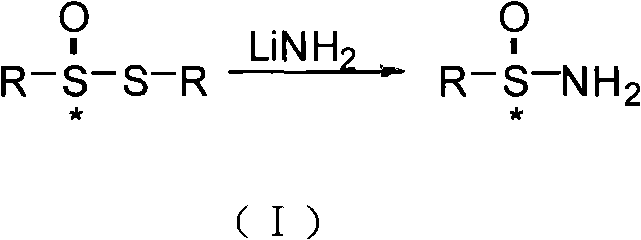Method for synthesizing chiral sulfenamide
A sulfenamide and chiral technology, which is applied in the field of synthesizing chiral sulfenamide, can solve the problems of cumbersome routes, increased costs, and high cost, and achieve the effects of wide application, low cost of ligands, and fewer reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0036] The present invention can be further illustrated by the following examples, but the present invention is not limited to the following examples.
[0037] In the present invention, the cheap and easy-to-obtain chloramphenicol derivatives and salicylaldehyde derivatives are firstly condensed to obtain the chiral ligand L with L1 or L2 structure * , and then use the method of chiral catalytic oxidation to oxidize the dialkyl (aryl) base disulfide into chiral thiosulfinate, and finally use the method of lithium amide reduction ammonolysis to obtain the chiral alkyl (aryl) base Sulfonamide, the steps are as follows:
[0038] 1. Preparation of ligands L1 and L2:
[0039] Preparation of Ligand L1:
[0040] Add 4.68g of 3,5-di-tert-butyl salicylaldehyde to a 250ml one-mouth bottle, dissolve it in 125mL of ethanol, add (1R,2S)-2-amino-1-(4'-nitrophenyl)-1,3 - 4.3 grams of propylene glycol, the solution immediately turned bright yellow, stirred overnight at room temperature, fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com