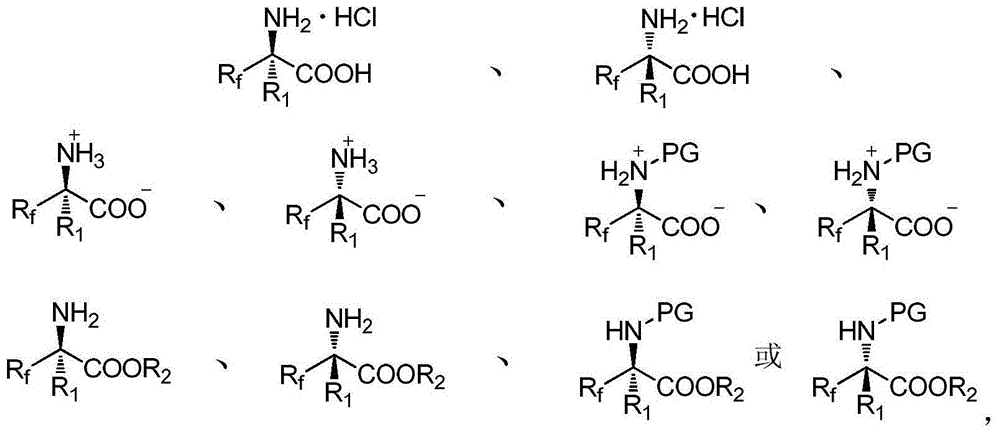
Alpha-fluoroalkyl-alpha-amino acid compound containing tetrasubstituted carbon chiral center and preparation method thereof
An amino acid and fluorine-containing alkyl technology, which is applied in the field of organic synthesis, can solve the problems of inability to obtain α-fluoroalkyl-α-amino acid compounds, etc., and achieves high yield, cheap and easily available raw materials, and good application prospects. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
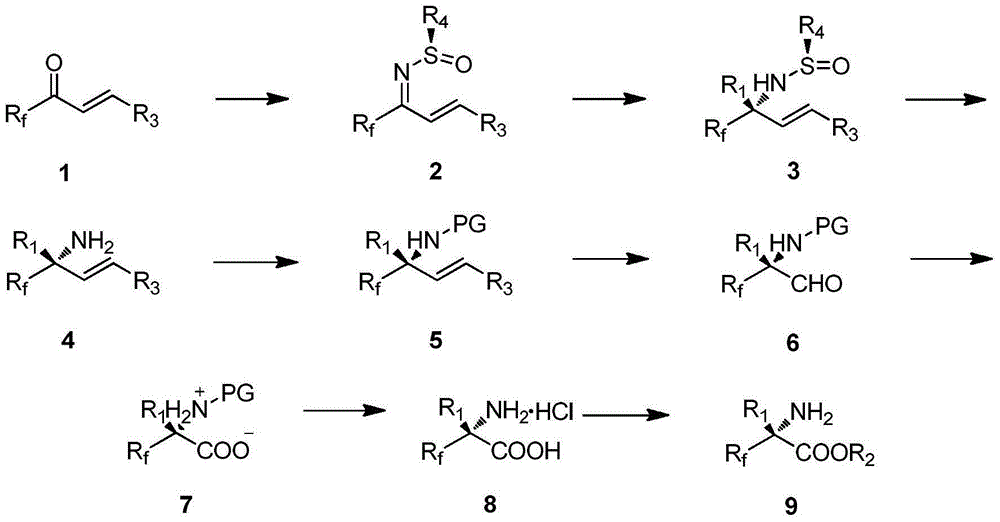
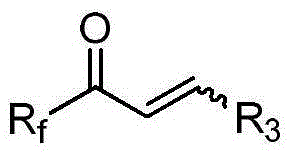
[0032] Dissolve fluorine-containing alkyl unsaturated ketone 1, chiral sulfenamide, and tetraethoxytitanium in an organic solvent, heat to reflux for 24 hours, quench the reaction, filter, separate and extract, dry, and column chromatography to obtain product 2 .
[0033] Further, the product 2 was dissolved in an organic solvent, under the protection of an inert gas, and an organolithium reagent was added under the condition of -40°C. After the reaction was completed, the reaction was quenched, liquid separation, extraction, drying, and column chromatography were performed to obtain the product 3.
[0034] Further, the product 3 was dissolved in an organic solvent, an aqueous solution of hydrogen chloride was added at room temperature, stirred at room temperature for 2 hours, and the solvent was spin-dried to obtain product 4.
[0035] Further, dissolve the product 4 in an organic solvent, add triethylamine, potassium carbonate, and benzoyl chloride, heat overnight for reacti...
Embodiment 2
[0051] Add 1g to the reaction bottle 666 mg 3g Ti(OEt) 4 , 45ml tetrahydrofuran, reflux at 80°C for 24 hours, cool to room temperature, add 30ml saturated sodium chloride aqueous solution, filter, wash the filter cake with ethyl acetate, combine the filtrate, separate liquid, extract with ethyl acetate, combine the organic phase, anhydrous Dried over sodium sulfate, filtered to remove the desiccant, spin-dried the solvent, and performed flash column chromatography (PE:EA=15:1) to obtain the product 1.3 g, 86% yield.
[0052] mp:55-56℃; [α] D 20 -985.76 (c=0.70, CHCl 3 ); FT-IR (KBr, cm -1 ):ν2964,2932,1617,1583,1451,1197,1144,1126,1079,977,762,698; 1 H NMR (400MHz, CDCl 3 ):δ8.00(d,J=16.8Hz,1H),7.44-7.47(m,2H),7.27-7.30(m,3H),7.23(d,J=16.8Hz,1H),1.25(s, 9H); 19 F NMR (376MHz, CDCl 3 ):δ-66.26(s,3F); 13 C NMR (CDCl 3): δ158.66(q, J=32.9Hz), 143.77(q, J=2.7Hz), 134.65, 130.86, 128.89, 128.35, 118.88(q, J=282.4Hz), 115.09, 60.58, 22.89.
[0053] Under argon prote...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com