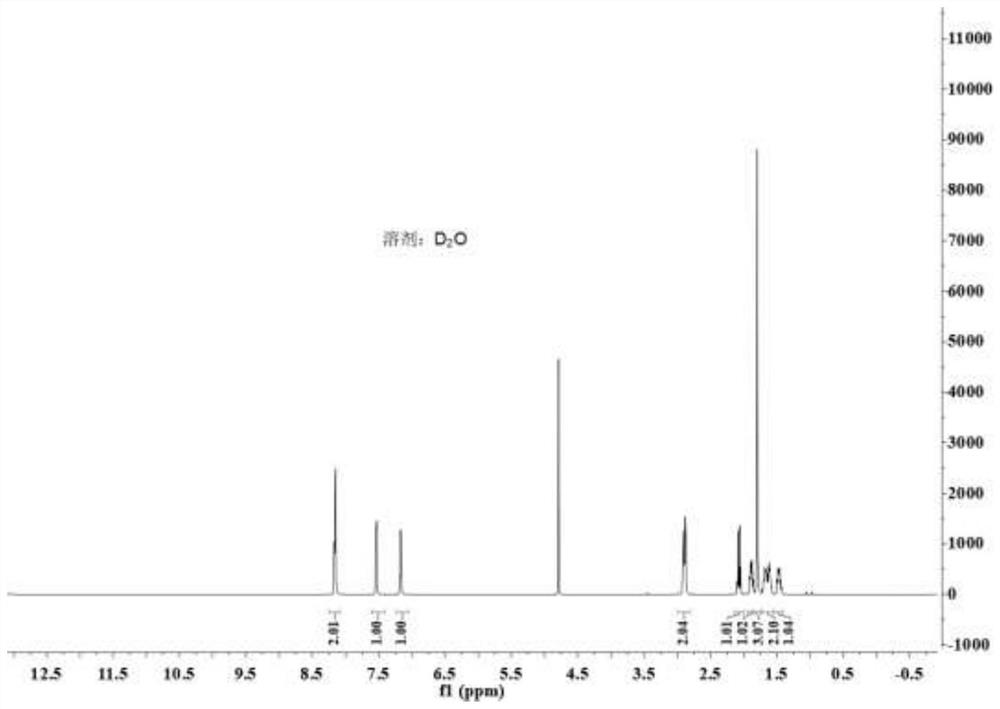
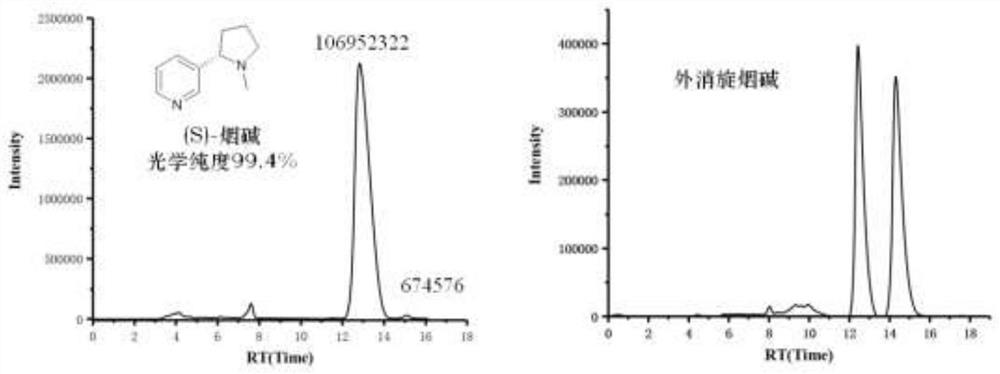
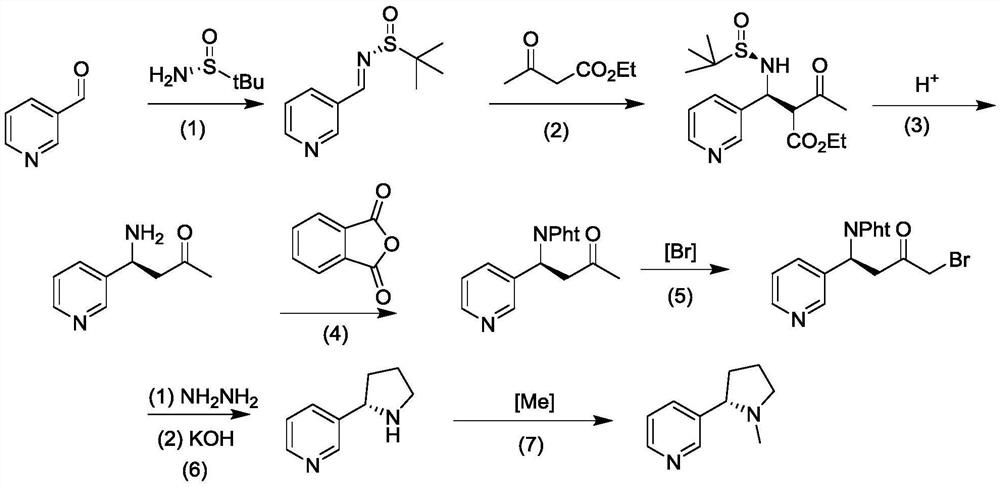
Method for asymmetrically synthesizing (S)-nicotine
An asymmetric and nicotine technology, applied in organic chemical methods, bulk chemical production, organic chemistry, etc., to achieve the effects of lower prices, lower production costs, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] A synthetic method for optically pure (S)-nicotine, the steps are as follows:
[0036] (1) Dissolve 3-pyridinecarbaldehyde (0.5mol) in ethanol (200mL), add (R)-tert-butylsulfinamide (0.55mol) and 0.60mol potassium hydrogensulfate, stir at room temperature for 3h, and depressurize Concentrate to remove ethanol, add 50mL water to the concentrated solution, and extract twice with ethyl acetate (150mL each time), combine the extracts and sequentially wash with dilute hydrochloric acid (0.1mol / L, 10mL dosage), water (25mL) and saturated salt Wash with water (15 mL), dry over anhydrous sodium sulfate (50 g), evaporate the solvent under reduced pressure, and obtain the product chiral imine;
[0037] (2) Under the ice bath, the chiral imine (0.1mol) obtained in the previous step was added to the ethanol (25mL) solution of the mixture of 0.1mol ethyl acetoacetate and 0.12mol sodium ethoxide, and the stirring reaction was continued under the ice bath until Thin-layer chromatogra...
Embodiment 2
[0044] A synthetic method for optically pure (S)-nicotine, the steps are as follows:
[0045] (1) Dissolve 3-pyridinecarbaldehyde (0.2mol) in ethanol (60mL), add 0.22mol of (R)-tert-butylsulfinamide and 38g of copper sulfate (0.24mol), stir at room temperature for 6h, and concentrate under reduced pressure 15mL of water was added to the concentrated solution, and extracted twice with ethyl acetate (25mL each time), the combined extracts were washed with dilute hydrochloric acid (0.1mol / L, 5mL), water (20mL) and saturated brine (10mL) successively , dried over anhydrous sodium sulfate (40 g). The solvent is evaporated under reduced pressure to obtain the product chiral imine, which can be directly used in the next reaction;
[0046] (2) Under ice bath, add the chiral imine (0.1mol) obtained in the previous step to the ethanol (50mL) solution of the mixture of 0.1mol ethyl acetoacetate and 0.12mol potassium tert-butoxide, and continue to stir under ice bath React until the thi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com