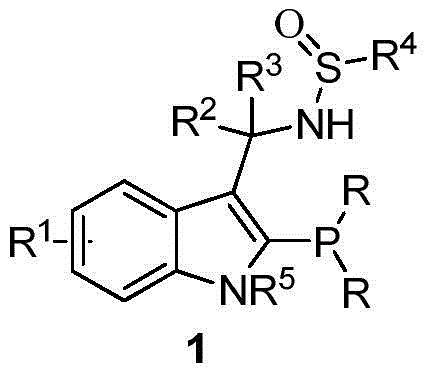
Indole framework based center chirality sulfonamides monophosphine ligand and preparation method
A sulfenamide and chiral technology, which is applied in the preparation of central chiral sulfenamide monophosphine ligands, ligands and their preparation fields, can solve the problems of long synthetic routes, harsh reaction conditions, expensive raw materials, etc., and achieve high Reactivity and stereoselectivity, good application prospects, easy to modify the effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
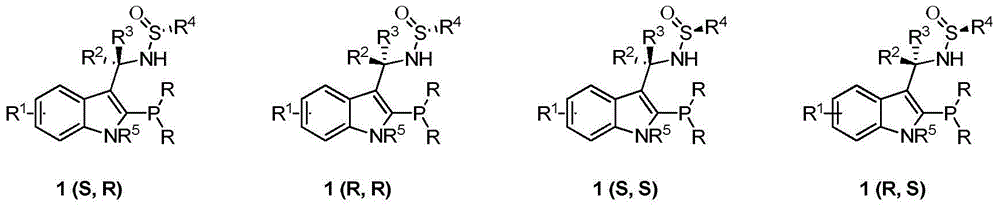
[0025] Example 1 (R)-N-((R)-(3-(2-(diphenylphosphino))-N-methyl-indolyl)(phenyl)methyl)-1-hydrogen- Synthesis of tert-butylsulfinamide [1a(R,R)]
[0026]
[0027] Among them, N 2 for nitrogen.
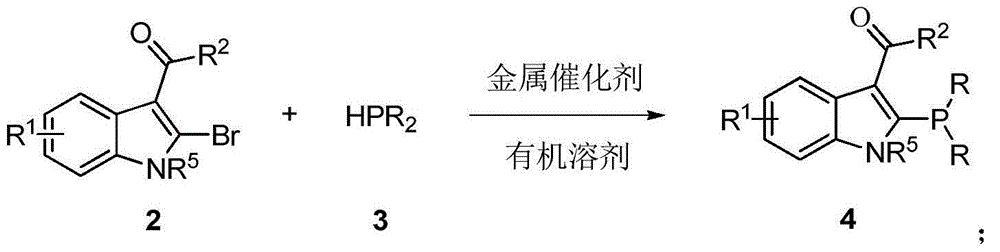
[0028] The first step: in a 500mL three-necked flask, add N-methyl-2-bromo-3-indole formaldehyde (50mmol) and diphenylphosphine (50mmol), add 150mL toluene under nitrogen atmosphere, add four Triphenylphosphinepalladium (2.5mmol), stirred at 50°C for 12h, the yield was 50%.
[0029]
[0030] The second step: in a 500mL three-necked flask, add N-methyl-2-diphenylphosphine-3-indolecarbaldehyde (50mmol) and (R)-(+)-tert-butylsulfinamide (50mmol ), added 150 mL of tetrahydrofuran under a nitrogen atmosphere, added tetraethyl titanate (100 mmol), stirred at 50° C. for 10 h, and the yield was 70%. 1 H NMR (400MHz, CDCl 3 ,ppm):δ=9.05(d,J=3.6Hz,1H),8.50(d,J=8.0Hz,1H),7.47–7.25(m,13H),3.53(s,3H),1.24(s, 8H). 13 C NMR (100MHz, CDCl 3 ,ppm):δ=158.16,157.99,141.56,141.24,140.15,133....
Embodiment 2
[0033] Example 2 (R)-N-((S)-(3-(2-(diphenylphosphino))-N-methyl-indolyl)(phenyl)methyl)-1-hydrogen- Synthesis of tert-butylsulfinamide [1a(R,S)]
[0034]
[0035] Other operations refer to Example 1, the nucleophile used is phenylmagnesium bromide, and the yield is 63%. 1 H NMR (400MHz, CDCl 3 , ppm): δ=7.79(d, J=8.1Hz, 1H), 7.51(t, J=8.4Hz, 4H), 7.42–6.99(m, 14H), 6.73(dd, J=8.2, 3.8Hz, 1H), 4.17(d, J=3.8Hz, 1H), 3.22(s, 3H), 1.19(s, 9H). 13 C NMR (100MHz, CDCl 3 ,ppm):δ=142.92,140.26,135.10,135.00,134.30,134.22,131.87,131.77,131.70,131.59,130.92,130.62,128.68,128.64,128.63,128.58,128.35,128.14,128.11,127.97,127.68,126.96, 126.88, 125.51, 125.44, 123.46, 121.59, 119.39, 109.64, 56.50, 56.28, 55.52, 32.46, 22.83. 31 P NMR (162MHz, CDCl 3 , ppm): δ=-34.99. IR (neat): 2920, 1456, 1056, 911, 741, 696; HRMS (ESI): calculated for C 32 h 33 N 2 OPS[M+Na] + :547.1943,found:547.1947.[α] D 20 =-125.9 (c=0.50, CHCl 3 ).
Embodiment 3
[0036] Example 3 (R)-N-((S)-(3-(2-(diphenylphosphino))-N-methyl-indolyl)(methyl)methyl)-1-hydrogen- Synthesis of tert-butylsulfinamide [1b(R,S)]
[0037]
[0038] Other operations refer to Example 1, the nucleophile is methylmagnesium bromide reagent, and the total yield is 70%. 1 H NMR (400MHz, CDCl 3 ,ppm): δ=7.87(d,J=8.1Hz,1H),7.46(d,J=7.6Hz,2H),7.40–7.21(m,8H),7.12(d,J=8.0Hz,1H) ,5.49(d,J=4.3Hz,1H),3.92–3.71(m,1H),3.24(d,J=1.5Hz,3H),1.71(d,J=6.7Hz,3H),1.11(s, 9H). 13 C NMR (100MHz, CDCl 3 ,ppm):δ=140.16,135.28,135.18,134.63,134.54,131.81,131.64,131.46,129.77,129.59,129.49,129.30,128.70,128.64,128.58,128.14,128.10,125.19,125.11,123.38,121.07,119.16, 109.72, 55.18, 49.30, 49.10, 32.33, 32.30, 24.96, 22.65. 31 P NMR (162MHz, CDCl 3 , ppm): δ=-34.98. IR (neat): 2959, 1433, 1362, 1052, 908, 740, 695; HRMS (ESI): calculated for C 27 h 31 N 2 OPS[M+Na] + :485.1787,found:485.1774.[α] D 20 =-117.6 (c=0.50, CHCl 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com