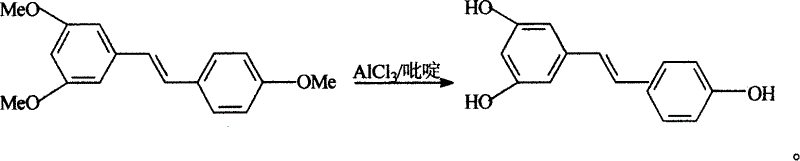Process for synthesizing resveratrol by using de-methylation technology
A technology of resveratrol and demethylation, applied in the fields of organic chemistry and functional food chemistry, can solve the problems of inconvenient industrialized production, use, transportation, storage, harsh reaction conditions, etc., and is conducive to labor protection, The effect of convenient procurement and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
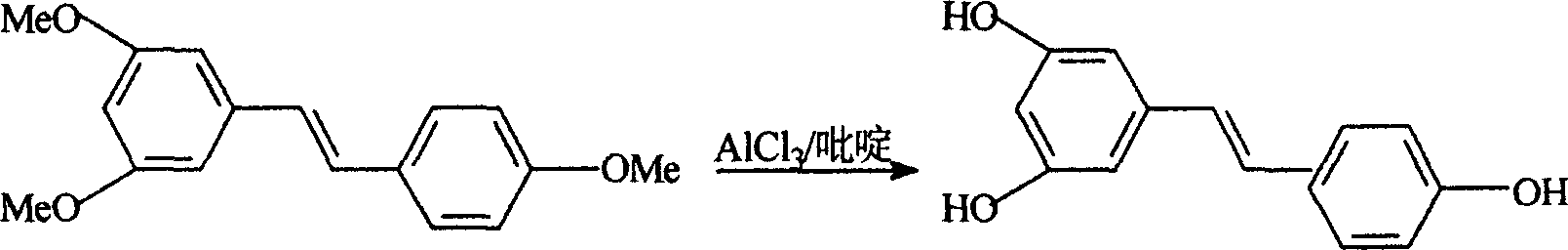
[0021] Example 1. Preparation of 3,5,4'-trihydroxyl-1,2-stilbene (resveratrol)
[0022] Add 30Kg 3,5,4'-trimethoxystilbene and 180Kg pure anhydrous pyridine into the reaction kettle, stir and dissolve, heat to raise the temperature slowly, control it at about 100°C, and add trichloride in batches Aluminum, a large amount of heat is released, the temperature is controlled not to exceed 170°C, and the addition of aluminum trichloride is completed in about 1 hour. After the addition, react at 150-165°C for 2-5 hours, complete the reaction time, and use high-performance liquid phase tracking to determine whether the demethylation is complete.
[0023] Pump 1200Kg of water into the hydrolysis kettle, pre-cool to 10°C, stir, put the above reaction solution into the hydrolysis kettle while it is hot under vacuum, and generate a lot of smoke, and remove the gas in time. The hydrolysis reaction solution was cooled to 10°C, centrifuged, and dried. Wash with 50Kg cold water and spin dr...
Embodiment 2
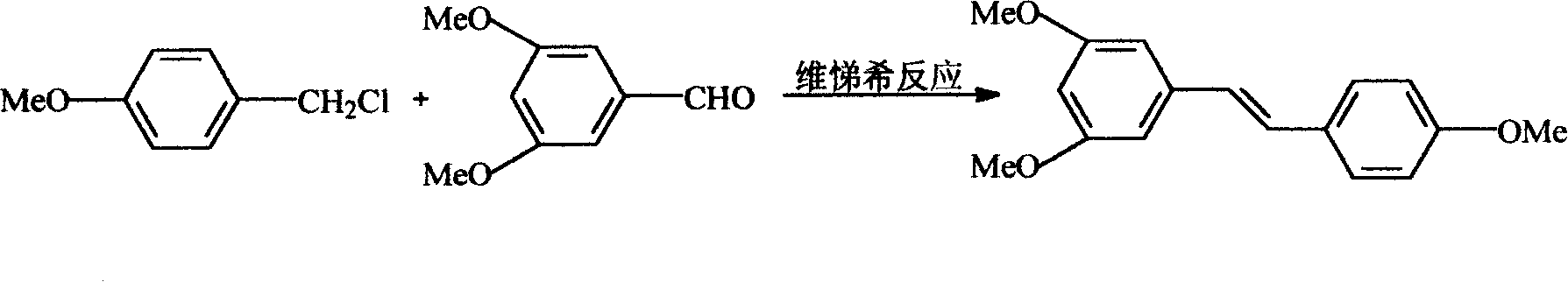
[0024] Example 2. Preparation of 3,5,4'-trimethoxystilbene
[0025] Add 64.3Kg p-methoxybenzyl chloride and 160Kg triethyl phosphite into the enamel reaction kettle respectively, start stirring, set the temperature of the oil bath at 140°C, react for 5-8 hours, and evaporate the triethyl phosphite in a vacuum. Add 400Kg DMF. Then add 48.7Kg of 50% sodium methoxide under cooling, react at 5°C for two hours until dissolved, then add 3,5-dimethoxybenzaldehyde into the reaction kettle, and react at room temperature for 10-12 hours. After the reaction is completed, about 98Kg of the intermediate can be obtained after treatment, with a yield of 90%, HPLC: ≥92%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com