Substituted cinnamide derivative, its preparation method and application
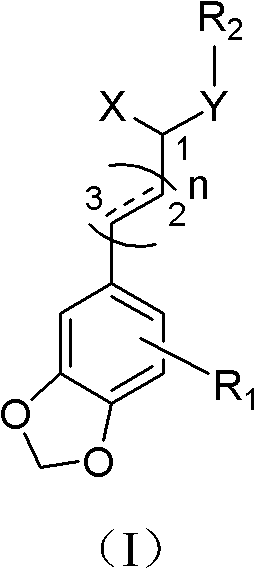
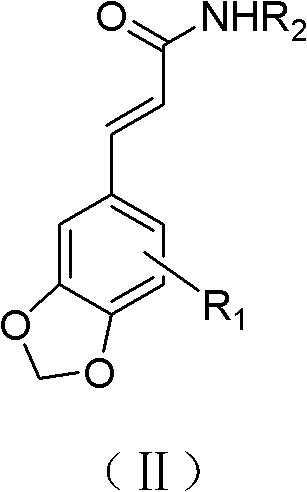
By synthesizing and screening 5'-methoxy-3',4'-methylenedioxycinnamic acid isobutylamide and its derivatives, the problems of toxic side effects and poor efficacy of existing antidepressant drugs have been solved and achieved It has significant antidepressant activity and minor side effects and is suitable for the treatment of depression.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
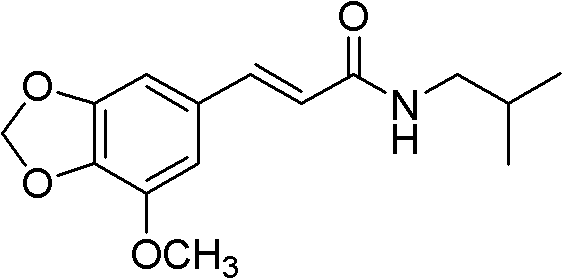
[0157] Example 1: 5'-methoxy-3', 4'-methylenedioxycinnamic acid isobutylamide (I-1)
[0158]
[0159] Add triethyl phosphonoacetate (300mg, 1.3mmol), 10ml anhydrous tetrahydrofuran, lithium hydroxide (163mg, 3.9mmol) in 50ml there-necked flask, N 2 Heated to 70°C under protection for 1 hour reaction. 3,4-Dioxymethylene-5-methoxybenzaldehyde (200mg, 1.1mmol) was dissolved in 5ml of anhydrous tetrahydrofuran, and it was dropped into the reaction flask within 0.5 hours. The reaction solution was reacted at 70° C. for 10 hours. TLC detection, stop heating after the reaction is complete. The reaction solution was concentrated to dryness by rotary evaporation, and 20 ml of distilled water was added to dissolve the solid. 2N hydrochloric acid was slowly added dropwise to the above solution to pH 2.0, and the stirring was continued for 1 hour, and a pale yellow solid was precipitated. The solid was collected by suction filtration under reduced pressure and dried in vacuo to obt...
Embodiment 2
[0164] Example 2: 5'-nitro-3', 4'-methylenedioxycinnamic acid isobutylamide (I-2)
[0165]
[0166] Add triethyl phosphonoacetate (300mg, 1.3mmol), 10ml anhydrous tetrahydrofuran, lithium hydroxide (163mg, 3.9mmol) in 50ml there-necked flask, N 2 Heated to 70°C under protection for 1 hour reaction. 3,4-Dioxymethylene-5-nitrobenzaldehyde (215mg, 1.1mmol) was dissolved in 5ml of anhydrous tetrahydrofuran, and it was dropped into the reaction flask within 0.5 hours. The reaction solution was reacted at 70° C. for 10 hours. TLC detection, stop heating after the reaction is complete. The reaction solution was concentrated to dryness by rotary evaporation, and 20 ml of distilled water was added to dissolve the solid. 2N hydrochloric acid was slowly added dropwise to the above solution until the pH was 2.0, and the stirring was continued for 1 hour, and a yellow solid was precipitated. The solid was collected by suction filtration under reduced pressure and dried in vacuo to o...
Embodiment 3
[0171] Example 3: 5'-iodo-3', 4'-methylenedioxycinnamic acid isobutylamide (I-3)
[0172]
[0173] Add triethyl phosphonoacetate (300mg, 1.3mmol), 10ml anhydrous tetrahydrofuran, lithium hydroxide (163mg, 3.9mmol) in 50ml there-necked flask, N 2 Heated to 70°C under protection for 1 hour reaction. 3,4-Dioxymethylene-5-iodobenzaldehyde (300mg, 1.1mmol) was dissolved in 5ml of anhydrous tetrahydrofuran, and it was dropped into the reaction flask within 0.5 hours. The reaction solution was reacted at 70° C. for 10 hours. TLC detection, stop heating after the reaction is complete. The reaction solution was concentrated to dryness by rotary evaporation, and 20 ml of distilled water was added to dissolve the solid. 2N hydrochloric acid was slowly added dropwise to the above solution to pH 2.0, and the stirring was continued for 1 hour, and a pale yellow solid was precipitated. The solid was collected by suction filtration under reduced pressure and dried in vacuo to obtain th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mass | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com