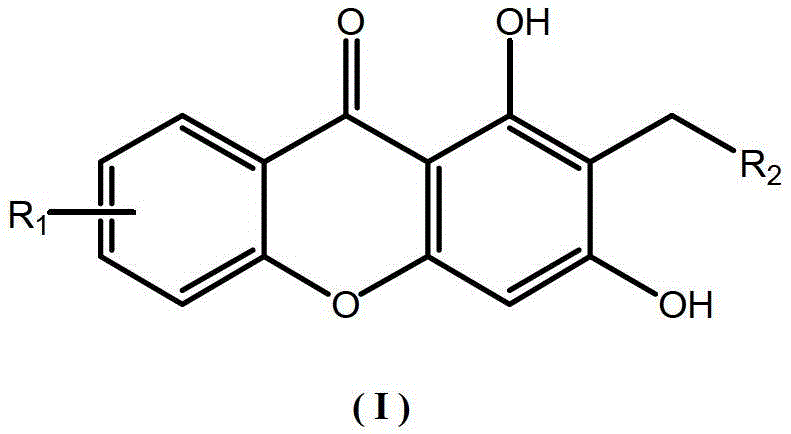
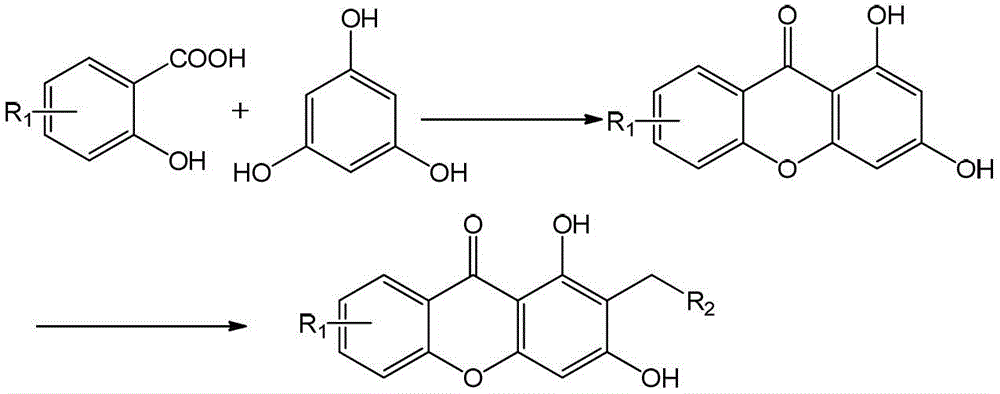
Xanthones and their antidepressant uses
A technology of xanthone and compound, applied to xanthone compound and its application field in antidepressant drugs, can solve problems such as large toxic and side effects, and achieve the effect of good antidepressant activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
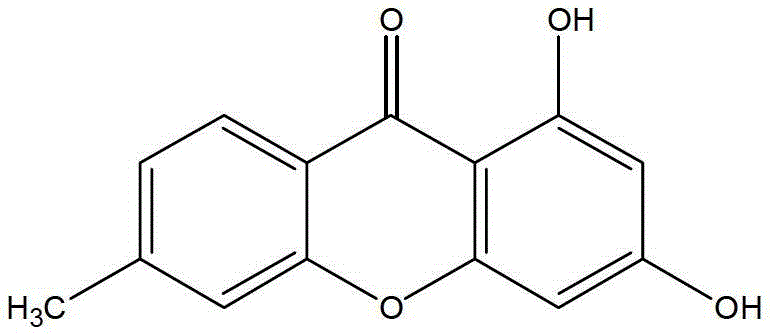
[0025] Example 1: 1,3-Dihydroxy-6-methyl-9H-xanthone
[0026]
[0027] Measure 100ml of phosphorus oxychloride, weigh 30g of zinc chloride, add to a 250ml three-neck flask, stir well and heat to 60°C, add 15.2g of 4-methylsalicylic acid and 18.4g of phloroglucinol after reacting for 1h (all dried), after the reaction temperature is stable, slowly heat to 70°C, take a sample after 3.5 hours of reaction, and detect by TLC (petroleum ether: ethyl acetate = 2:1), the reaction is complete, stop the reaction, pour it after cooling completely Add about 1500ml of ice water, stir, let stand, filter with suction, wash until neutral, filter cake is dried and separated by silica gel column chromatography (petroleum ether: ethyl acetate = 1:1), to obtain 10.8g of light yellow powder, product The rate is 44.6%.
[0028] ESI-MSm / z:241.0[M - ]. 1 HNMR (CDCl 3 +DMSO-d 6 , 500MHz) δ: 12.89(1H, s), 8.05(1H, d, J=8.5Hz), 7.21(1H, s), 7.16(1H, d, J=8Hz), 6.37(1H, d, J=8Hz) 2Hz), 6.26 (1H,...
Embodiment 2
[0029] Example 2: 1,3-Dihydroxy-7-methyl-9H-xanthone
[0030]
[0031] 15.2g of 5-methylsalicylic acid and 18.4g of phloroglucinol were obtained according to the synthetic method of Example 1 to obtain 14.6g of light yellow powder with a yield of 60.3%.
[0032] ESI-MSm / z:241.0[M - ]. 1 HNMR (CDCl 3 +DMSO-d 6 , 500MHz) δ: 12.87(1H, s), 10.41(1H, s), 7.96(1H, d, J=1Hz), 7.54(1H, dd, J=8.5, 2Hz), 7.34(1H, d, J =8.5Hz), 6.37(1H, d, J=2Hz), 6.23(1H, d, J=2Hz), 2.47(3H, s).
Embodiment 3
[0033] Example 3: 1,3-dihydroxy-5-methoxy-9H-xanthone
[0034]
[0035] 16.8g of 4-methoxysalicylic acid and 18.4g of phloroglucinol were obtained according to the synthetic method of Example 1 to obtain 9.4g of light yellow powder with a yield of 36.4%.
[0036] ESI-MSm / z:256.9[M - ]. 1 HNMR (DMSO-d 6 , 500MHz) δ: 12.80(1H, s), 11.05(1H, s), 7.64(1H, dd, J=8, 1.5Hz), 7.54(1H, d, J=8Hz), 7.36(1H, d, J=8Hz), 6.40 (1H, d, J=2Hz), 6.22 (1H, d, J=2Hz), 3.97 (3H, s).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com