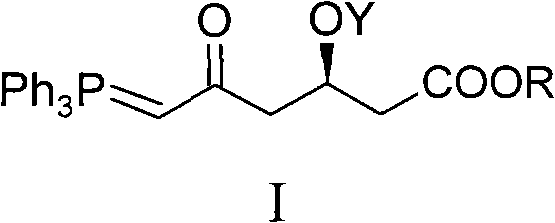
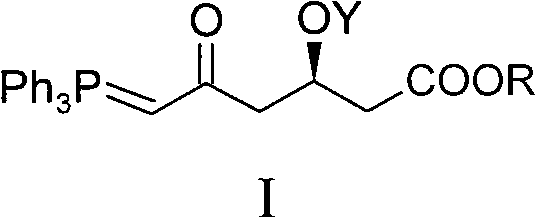
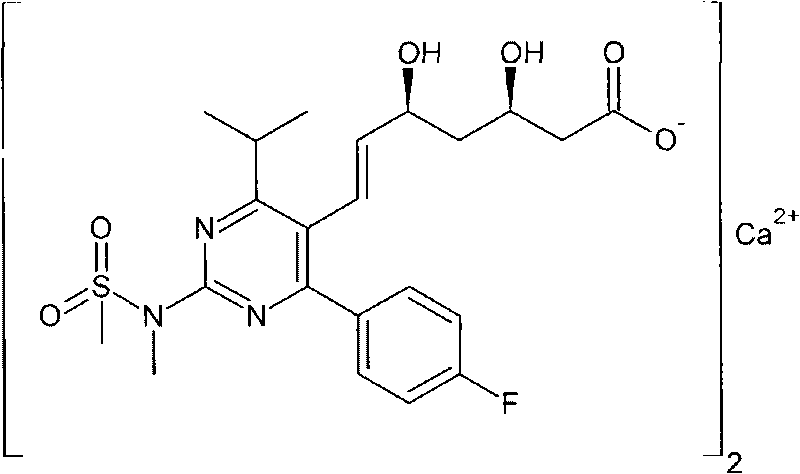
Method for preparing rosuvastatin calcium midbody
A technology for rosuvastatin calcium and intermediates, which is applied in the field of preparation of pharmaceutical intermediates, can solve the problems of high price of raw material anhydride compounds, low yield of reaction products in the first step, unsuitability for large-scale production, etc., and achieve cheap raw materials , mild conditions, and the effect of three wastes treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1, the preparation of compound I-1 (R is Me in general formula I, and Y is TBS)
[0040]
[0041] a. Reaction of vinyl chloride with metal magnesium to produce vinyl chloride Grignard reagent, and then carry out Grignard reaction with R-epichlorohydrin under the catalysis of cuprous chloride to obtain (2R)-1-chloro-2-hydroxyl -4-pentene;
[0042]
[0043] Add 2700ml of anhydrous-treated tetrahydrofuran and 216g of magnesium powder into the reaction flask, stir evenly, then add 4.5g of iodine, and add 100g of 1,2-dibromoethane in several times, after the addition, slowly heat up to 60-64 ℃, heat preservation and stirring for 3 to 4 hours, then feed vinyl chloride gas for reaction, react at a temperature of 60 to 64 ℃ and continue to feed vinyl chloride gas for 10 to 11 hours, then stop the reaction, cool down to 25 to 35 ℃, measure The concentration of the obtained vinyl chloride Grignard reagent is 3.34mol / L;
[0044] Continue to cool the reaction sol...
Embodiment 2
[0061] Embodiment 2, the preparation of compound I-1
[0062] a. Reaction of vinyl chloride with metal magnesium to produce vinyl chloride Grignard reagent, and then carry out Grignard reaction with R-epichlorohydrin under the catalysis of cuprous chloride to obtain (2R)-1-chloro-2-hydroxyl -4-pentene;
[0063] Add 300ml of anhydrous-treated tetrahydrofuran and 24g of magnesium powder into the reaction flask, stir evenly, then add 0.5g of iodine, and add 11g of 1,2-dibromoethane several times. ℃, heat preservation and stirring for 3 to 4 hours, then feed vinyl chloride gas for reaction, react at a temperature of 60 to 64 ℃ and continue to feed vinyl chloride gas for 10 to 11 hours, then stop the reaction, cool down to 25 to 35 ℃, measure The concentration of the obtained vinyl chloride Grignard reagent is 3.13mol / L;
[0064] Continue to cool the reaction solution to -35~-25°C, add 35g cuprous chloride, then slowly add 280g R-epichlorohydrin (the molar ratio of cuprous chlori...
Embodiment 3
[0076] Embodiment 3, the preparation of compound I-1
[0077] a. Reaction of vinyl chloride with metal magnesium to produce vinyl chloride Grignard reagent, and then carry out Grignard reaction with R-epichlorohydrin under the catalysis of cuprous chloride to obtain (2R)-1-chloro-2-hydroxyl -4-pentene;
[0078] Add 2700ml of anhydrous-treated tetrahydrofuran and 216g of magnesium powder into the reaction flask, stir evenly, then add 4.5g of iodine, and add 100g of 1,2-dibromoethane in several times, after the addition, slowly heat up to 60-64 ℃, heat preservation and stirring for 3 to 4 hours, then feed vinyl chloride gas for reaction, react at a temperature of 60 to 64 ℃ and continue to feed vinyl chloride gas for 10 to 11 hours, then stop the reaction, cool down to 25 to 35 ℃, measure The concentration of gained vinyl chloride Grignard reagent is 3.42mol / L;
[0079] Continue to cool the reaction solution to -35~-25°C, add 40g of cuprous chloride, then slowly add 370g of R-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com