Conjugation arborization electroinduced ethereal blue light material, preparation method and application thereof
A dendritic and conjugated technology, applied in the direction of luminescent materials, chemical instruments and methods, circuits, etc., can solve the problems of insufficient luminous brightness and color purity of blue light materials, restrictions on the development of polyfluorene materials, poor solubility and stability, etc. Achieve the effects of reducing fluorescence quenching, improving efficiency and color purity, and increasing solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
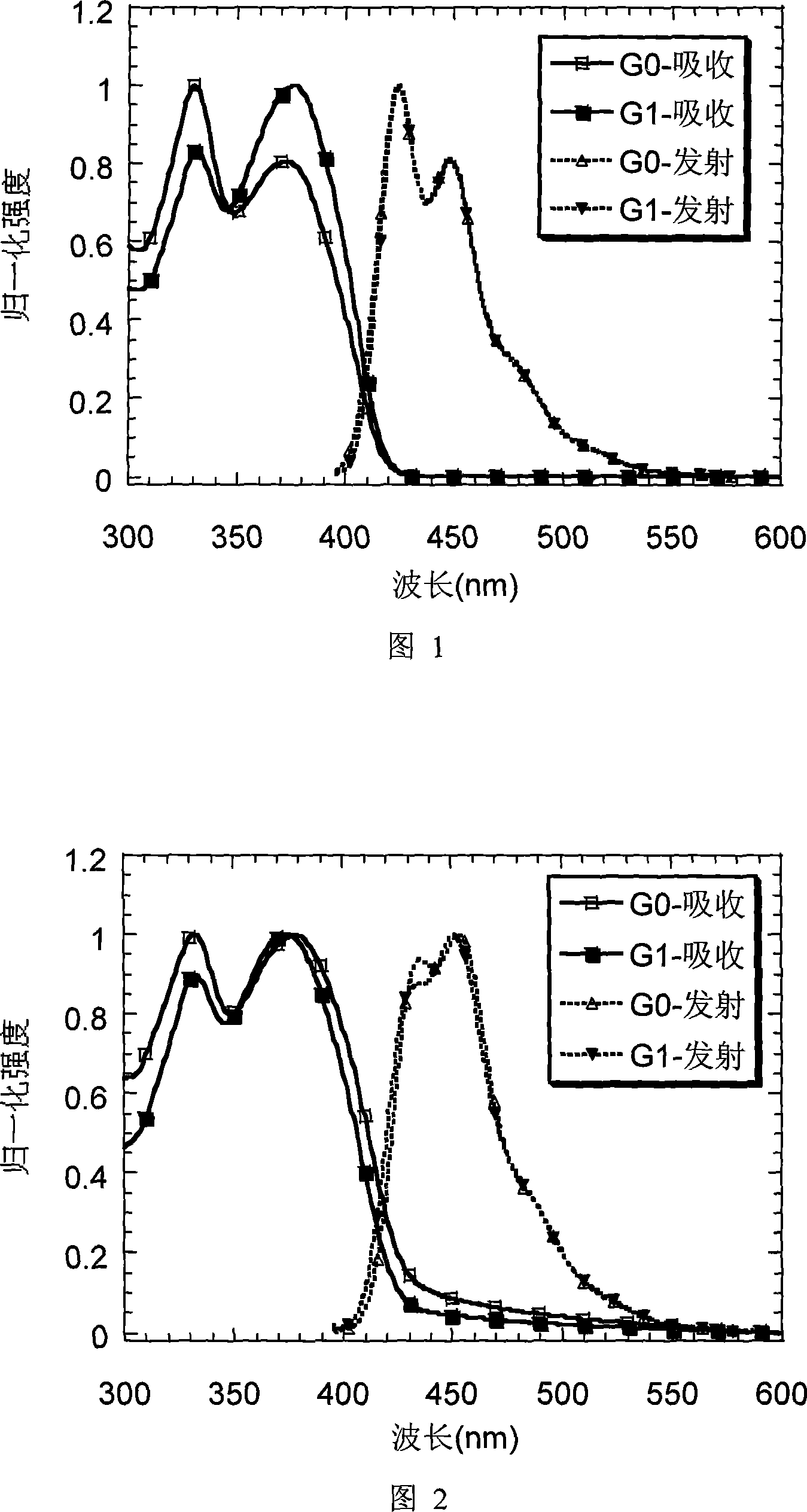
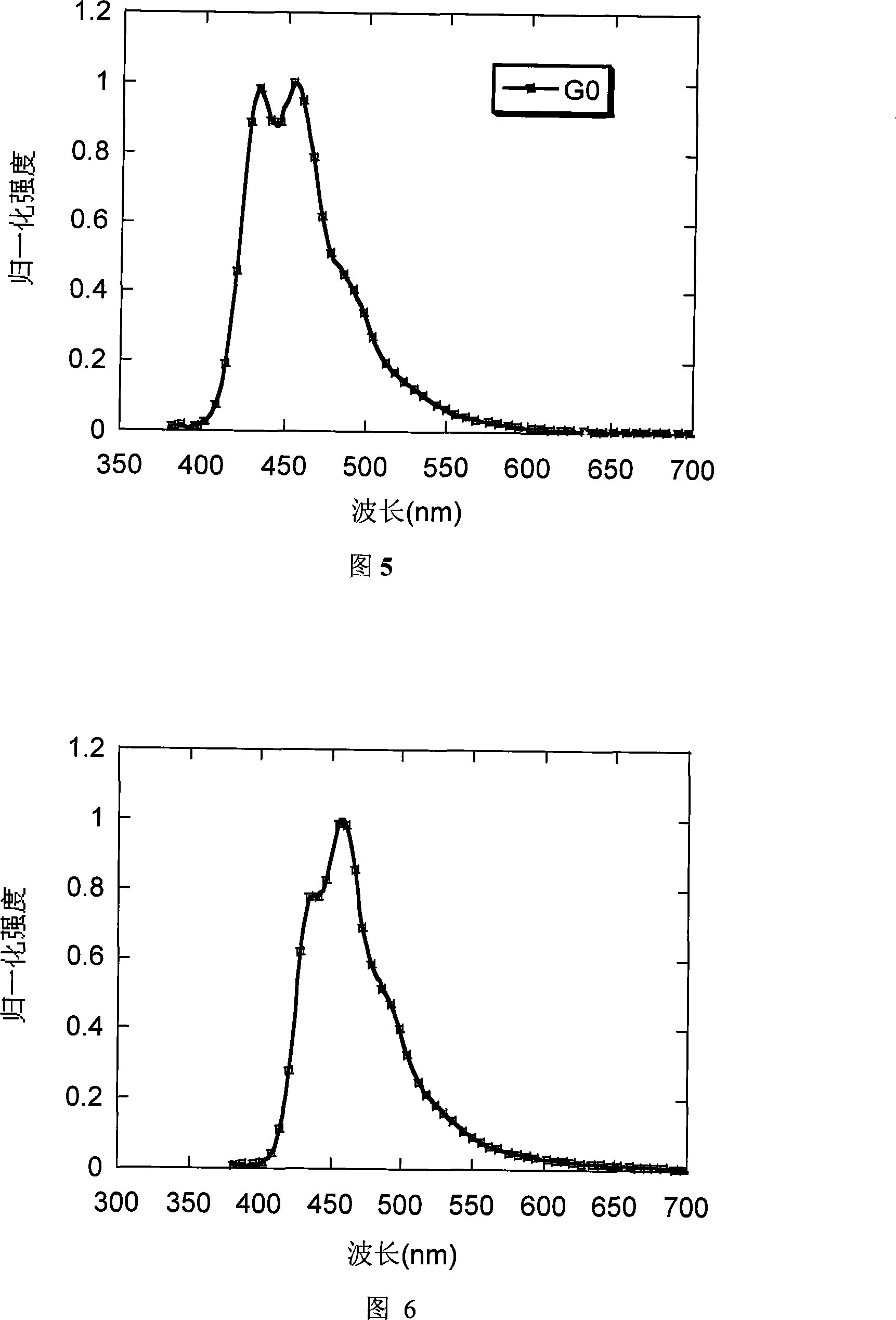
[0054] Embodiment 1: Preparation of G0 and its property determination
[0055] Horner-Wadsworth-Emmons reaction of the triphosphonate 5 shown below with the monoaldehyde derivative 7 of trisindene affords GO. The synthesis of triphosphonates, monoaldehyde derivatives of tripolyindene and GO is shown below:
[0056]
[0057] (1) Preparation of compounds 2 and 6:
[0058] Under nitrogen protection, 2.21g (1.1eq., 14.72mmol) 4-formylphenylboronic acid, 20g tribromide 1 (1.0eq., 18.40mmol), 10mg Pd(PPh 3 ) 4 Add 75 mL THF and 68 mL 2M Na 2 CO 3 In aqueous solution, reflux for 10 hours. Cool, extract the organic phase, NH 4 Washed with saturated aqueous Cl solution, anhydrous Na 2 SO 4 dry. The solvent was removed under reduced pressure, and the solid was separated through a silica gel column, and the developing solvent was petroleum ether / ethyl acetate=20 / 1~10 / 1 (volume ratio), and yellow solid trialdehyde 2 (9%) and monoaldehyde 6 ( 34%). Trialdehyde 2: 1 H NMR (CD...
Embodiment 2
[0071] Embodiment 2: Preparation of G1 and its property determination
[0072] Next-generation monoaldehydes undergo Horner-Wadsworth-Emmons reactions with triphosphonates to give G1. The synthesis of next-generation monoaldehydes and G1 is shown below:
[0073]
[0074] (1) Preparation of compound 8:
[0075] Dialdehyde 8 was prepared together with trialdehyde 2 and monoaldehyde 6, and the yield of dialdehyde 8 was 23%. 1 HNMR (CDCl 3 , 300MHz, ppm): δ10.11(2H, s), 8.44(2H, m), 8.26(1H, d, J=9.0Hz), 8.03(4H, d, J=8.5Hz), 7.93(4H, d, J=8.5Hz), 7.74-7.72(4H,m), 7.61(1H,s), 7.55(1H,d,J=9.0Hz), 3.04-2.89(6H,m), 2.21-2.05(6H , m), 1.02-0.77 (36H, m), 0.63-0.50 (30H, m). 13 C NMR (CDCl 3 , 75MHz, ppm): δ191.9, 155.9, 154.4, 147.2, 145.9, 145.6, 145.1, 140.5, 139.0, 138.0, 137.9, 137.8, 137.6, 135.1, 131.2, 130.9, 130.3, 129.7, 1289.5, 12 126.6, 126.2, 126.0, 125.6, 125.1, 124.4, 121.4, 121.0, 120.2, 55.9, 37.7, 37.1, 36.8, 36.1, 31.4, 29.4, 23.9, 22.2, 13.8. MALDI-TOF MS ...
Embodiment 3
[0090] Embodiment 3: device making
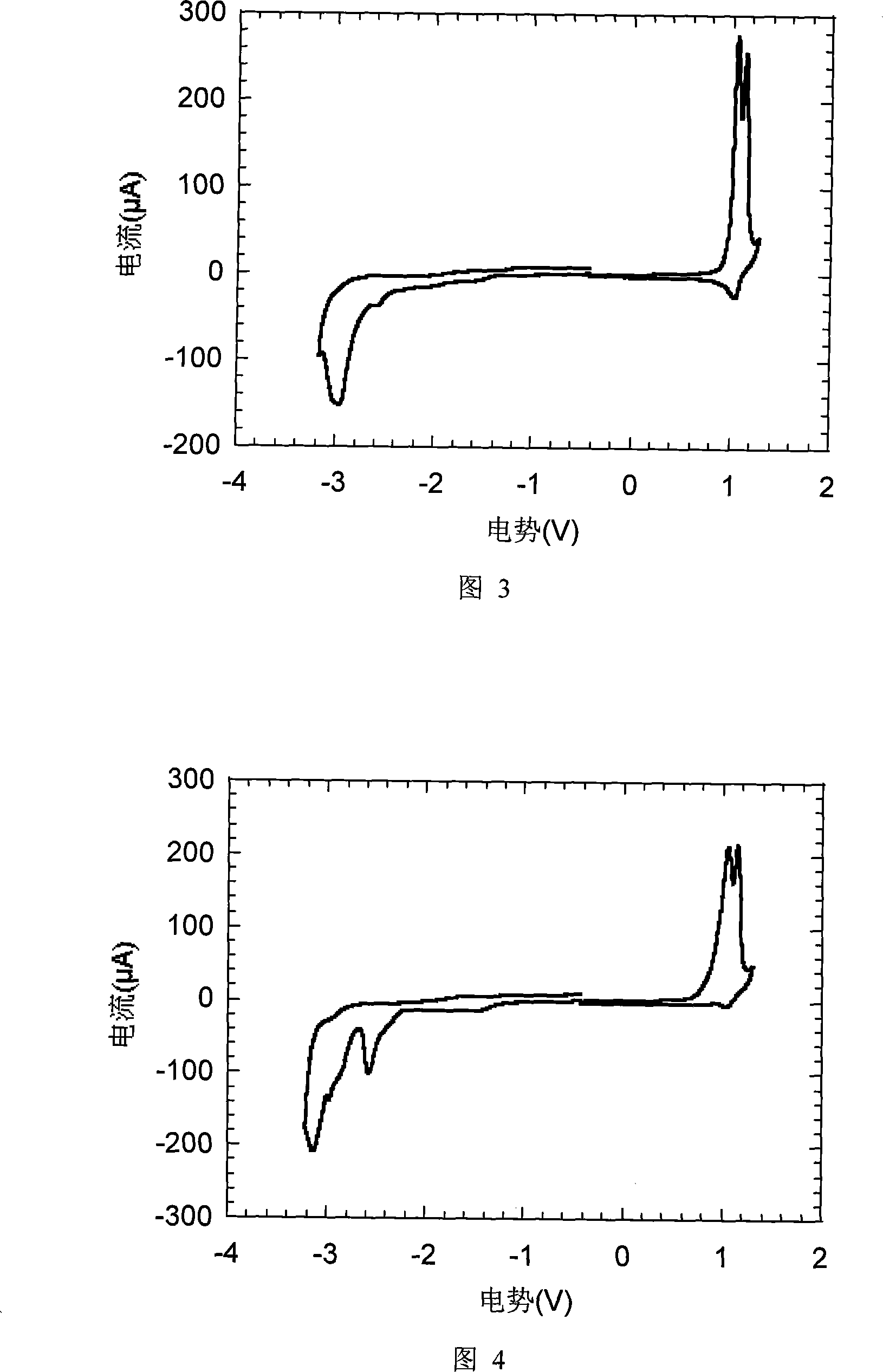
[0091] The typical device manufacturing process of the present invention is as follows: ITO (indium tin oxide) glass is ultrasonicated for ten minutes with acetone, alkaline washing solution, pure water (twice), and isopropanol, and then treated with ozone plasmar for 5 minutes. The hole injection layer PEDOT (poly(3,4-ethylenedioxythiophene)) was spin-coated on the treated substrate to form a film with a thickness of 50 nm, and was heated in air at 160° C. for 6 minutes. A hole transport layer PVK (poly(9-vinylcarbazole)) (thickness 40 nm) was spin-coated thereon, and heated for 15 minutes in a nitrogen atmosphere. Then spin-coat the light-emitting layer (G0 or G1, thickness 60nm), and heat for 15 minutes in a nitrogen atmosphere. Finally, Ba / Al (with a thickness of 4.5nm / 150nm) is vacuum evaporated to complete the device. The device structure is ITO / PEDOT(50nm) / PVK(40nm) / G0 or G1(60nm) / Ba(4.5nm) / Al(150nm). The starting voltage of the G...
PUM
| Property | Measurement | Unit |
|---|---|---|
| external quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com