Methyl pyridinium with organic two-photon absorption materia, and preparation method and application thereof
A technology of two-photon absorption and picoline salt, which is applied in the fields of color-changing fluorescent materials, organic chemistry, chemical instruments and methods, and can solve rare problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
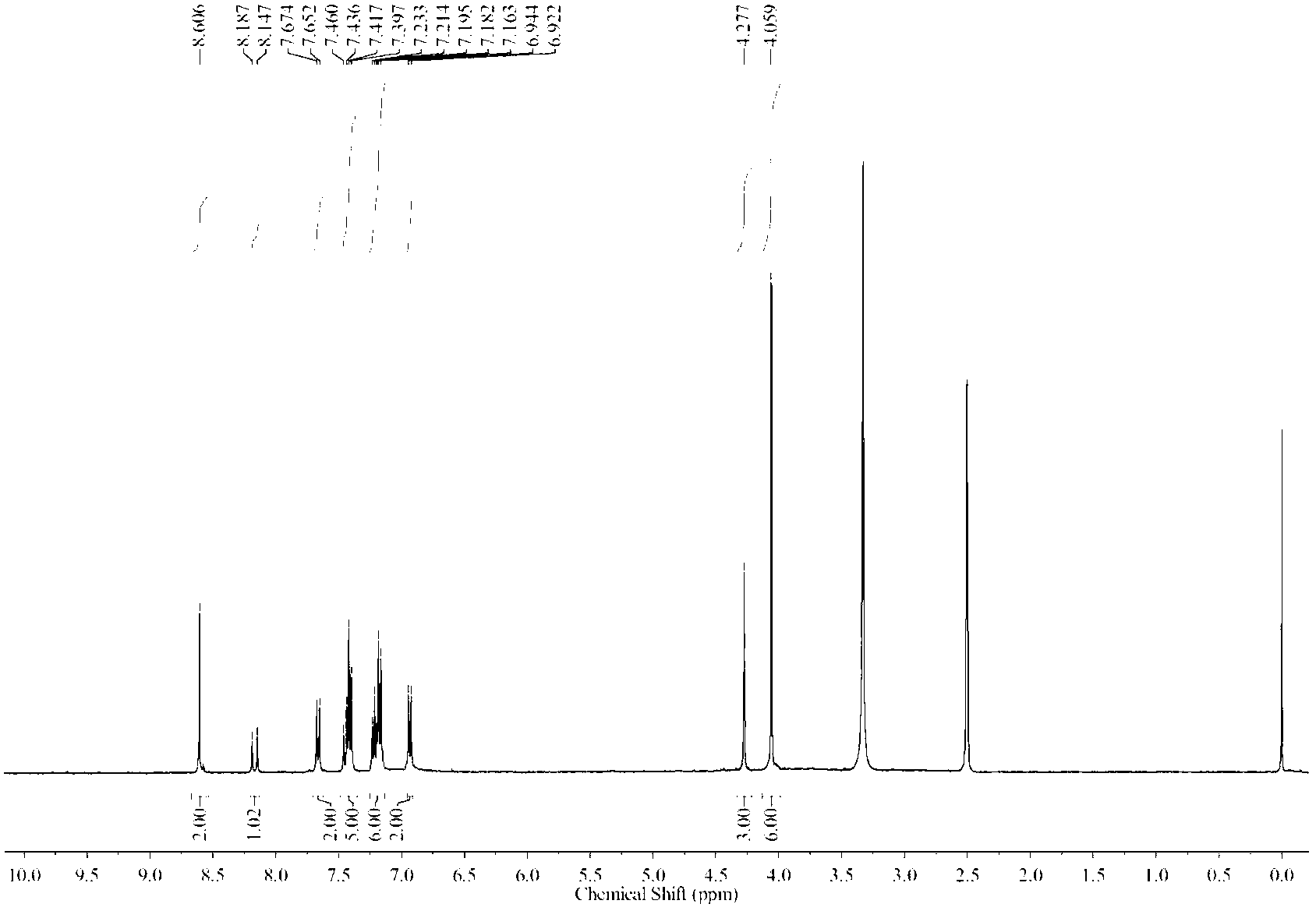
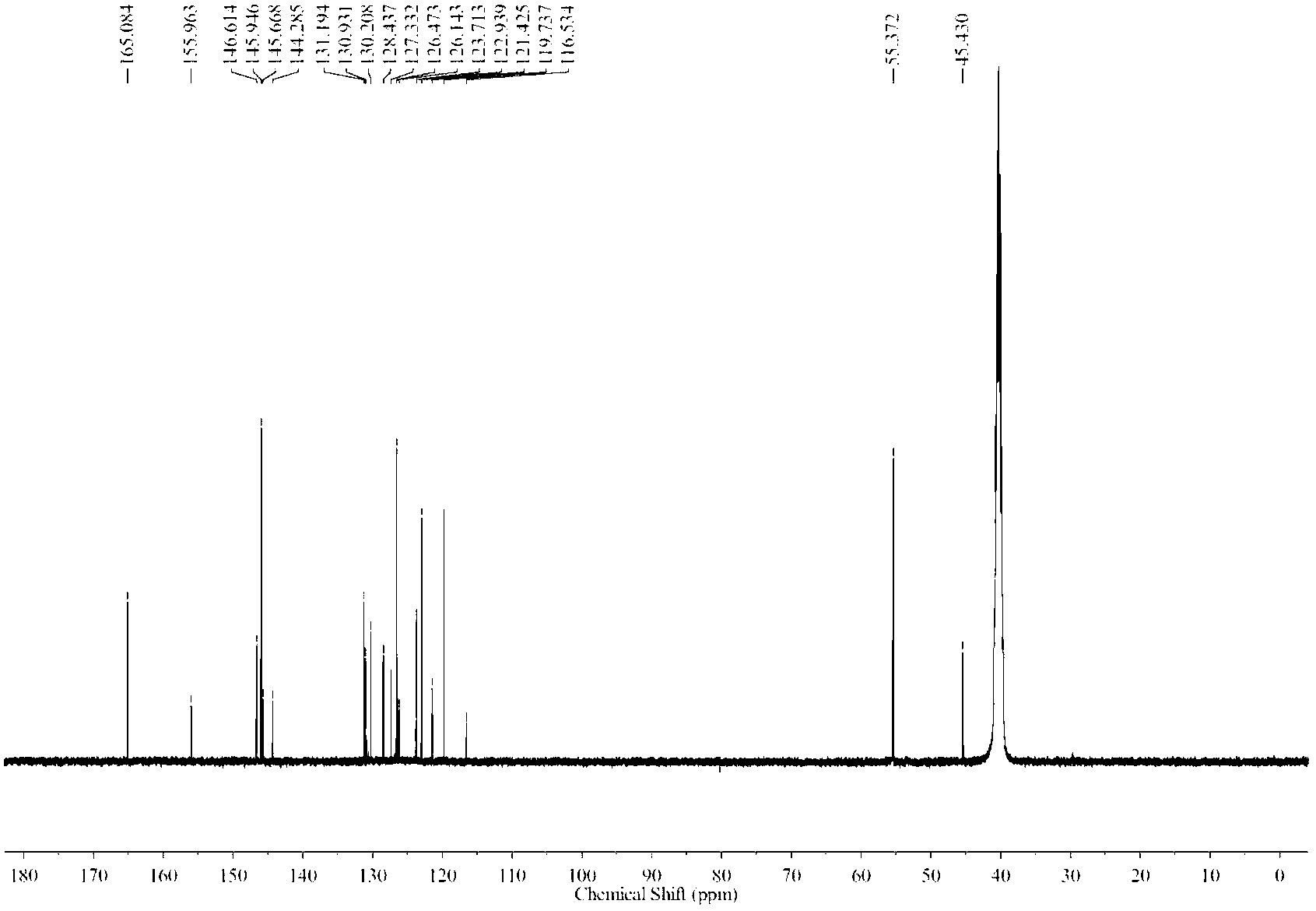
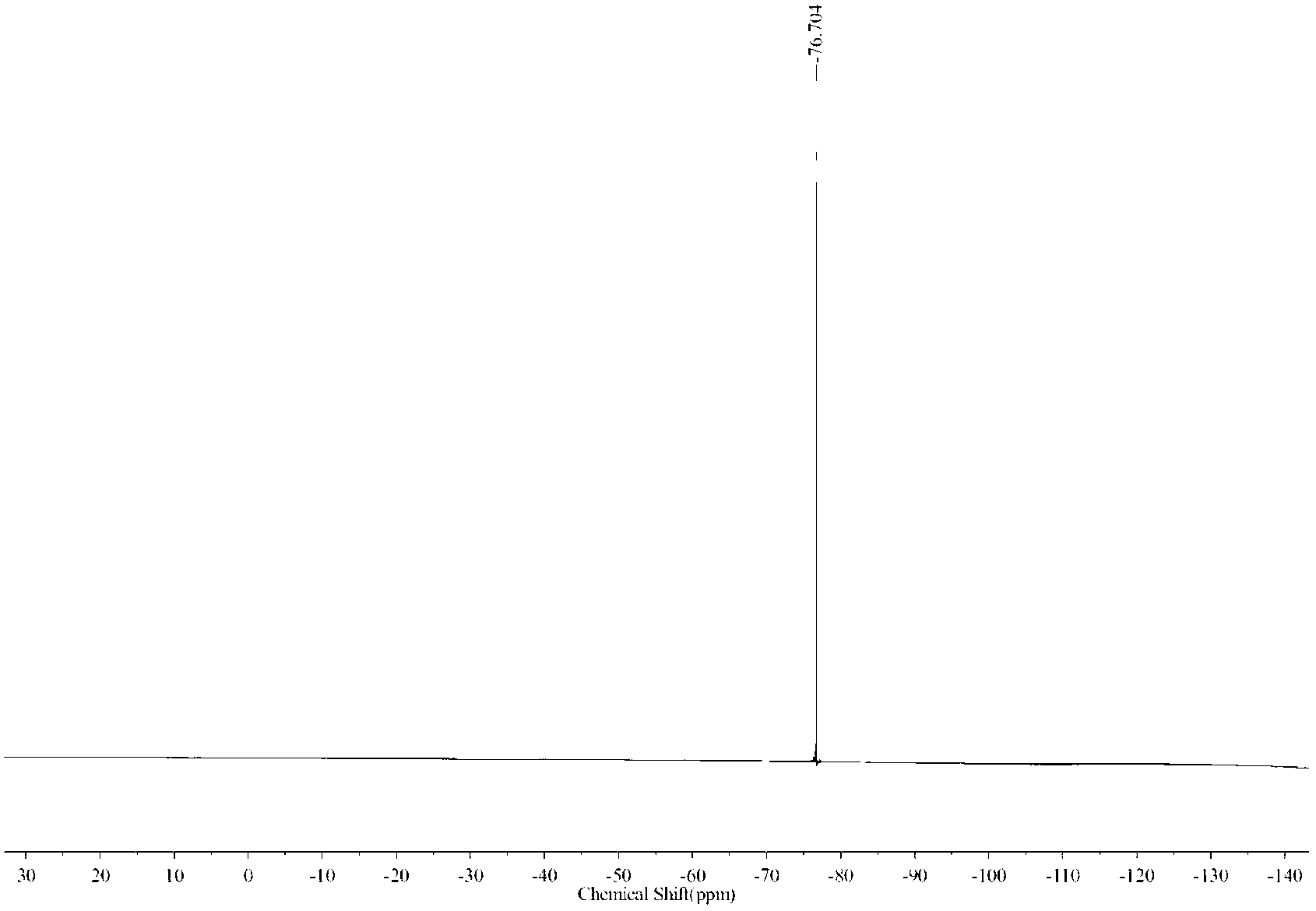
Image
Examples
Embodiment 1
[0090] Example 1: Preparation of N-methyl-(E)-4-[4'-(dianilino styryl)]pyridine-2,6-dicarboxylic acid dimethyl trifluoromethanesulfonate
[0091] (1) Preparation of pyridine-2,6-dicarboxylic acid
[0092]
[0093] Add 350 mL of distilled water, 41.0 g (261 mmol) of potassium permanganate and 5.0 g (46 mmol) of 2,6-lutidine into a 500 mL round bottom flask. Boiling and reflux reaction for 3 hours. Cool and filter, concentrate under reduced pressure to one-fifth of the original volume, and add 10mL of 70% sulfuric acid while hot. Cool naturally and filter to obtain crude product. Recrystallized twice from water to obtain 7.3 g of white solid. Yield: 98.6%. Mp: 229-230°C.
[0094] (2) Preparation of dimethyl pyridine-2,6-dicarboxylate
[0095]
[0096] Add 31.0g (190mmol) pyridine-2,6-dicarboxylic acid into a 500mL round bottom flask, coat the lower end of the spherical condenser tube with vacuum silicone ester, insert the spherical condenser tube, and pour 120mL (165...
Embodiment 2
[0112] Example 2: 4-(N-methyl-2,6-dimethoxycarbonyl-pyridine-4-(E)-vinyl)-4'-(2,6-dimethoxycarbonyl-pyridine- Preparation of 4-(E)-vinyl)triphenylamine trifluoromethanesulfonate
[0113] (1) The preparation method of 4-methylenetriphenylphosphoryl-pyridine-2,6-dicarboxylate is the same as in Example 1.
[0114] (2) Preparation of 4,4'-bis(2,6-dimethoxycarbonyl-pyridine-4-(E)-vinyl)triphenylamine
[0115]
[0116] Add 242.8mg (0.48mmol) chloride-4-methylenetriphenylphosphoryl-pyridine-2,6-dicarboxylic acid dimethyl ester, 60.3mg (0.20mmol) bis(4-methyl) into a 50mL round bottom flask Acylphenyl) aniline, nitrogen protection. Inject 20.0 mL of absolute methanol with a 20.0 mL syringe. Cool in an ice-water bath and stir at medium speed. Use a 5.0mL syringe to slowly inject 5.0mL (123.60mmol) of an absolute methanol solution containing 21.61mg (0.40mmol) of sodium methoxide into the flask. After the dropwise addition, the ice-water bath was removed, and the stirring reacti...
Embodiment 3
[0120] Example 3: Preparation of 4,4'-bis(N-methyl-2,6-dimethoxycarbonyl-pyridine-4-(E)-vinyl)triphenylamine bis-trifluoromethanesulfonate
[0121] (1) The preparation method of 4,4'-bis(2,6-dimethoxycarbonyl-pyridine-4-(E)-vinyl)triphenylamine is the same as in Example 2.
[0122] (2) Preparation of 4,4'-bis(N-methyl-2,6-dimethoxycarbonyl-pyridine-4-(E)-vinyl)triphenylamine bis-trifluoromethanesulfonate
[0123]
[0124] Add 136.6mg (0.2mmol) 4,4'-bis(2,6-dimethoxycarbonyl-pyridine-4-(E)-vinyl)triphenylamine into a 50mL round-bottomed flask, and protect it with nitrogen. Inject 8.0 mL of dichloromethane with a 10.0 mL syringe. Under an ice-water bath, use a 1.0mL syringe to inject a dichloromethane solution containing 0.04mL methyl trifluoromethanesulfonate 1.0mL (0.5mmol). Stir at medium speed for 72 hours at 40°C. The reaction was cooled to room temperature, diluted with 50 mL of anhydrous ether, and cooled overnight in a refrigerator. Suction filtration, discard the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com