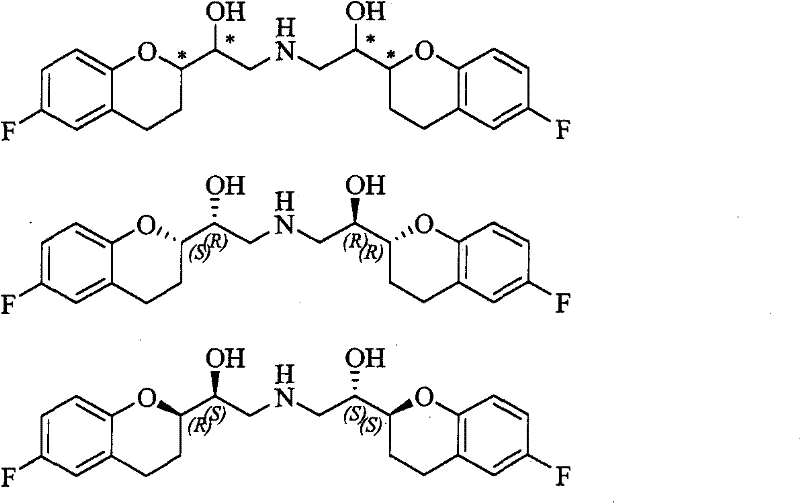
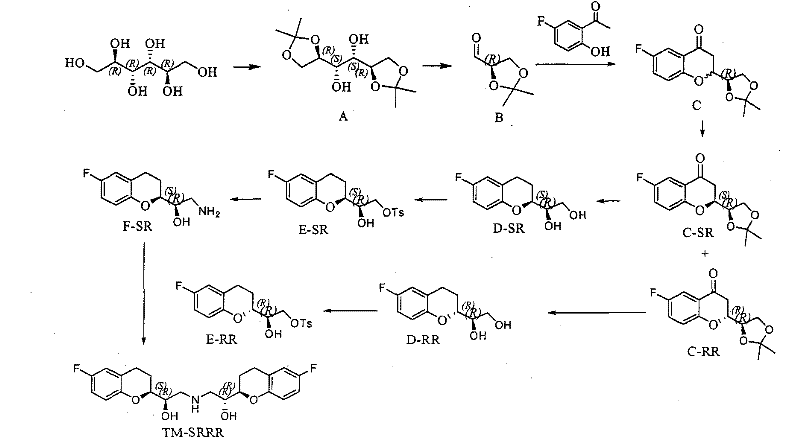
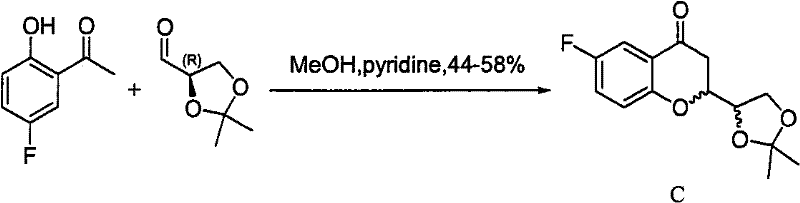
Method for preparing nebivolol hydrochloride
A compound, the technology of dichloromethane, applied in the field of preparing nebivolol hydrochloride, can solve the problems of harsh reaction conditions, high cost, and many synthesis steps, and achieve mild reaction conditions, low price, and cheap and easy-to-obtain raw materials Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0047] The present invention will be further described below through specific implementation in the form of examples. It should be understood that the preparation methods of the examples of the present invention are only used to illustrate the present invention, rather than limit the present invention, and the simple improvements to the preparation methods of the present invention under the premise of the concept of the present invention all belong to the protection scope of the present invention.
[0048] 1. (R, R)-2-(2',2'-dimethyl-[1,3]-dioxol-4-yl)-6-fluoro-4-chromanone and (S, Preparation of R)-2-(2', 2'-dimethyl-[1,3]-dioxolan-4-yl)-6-fluoro-4-chromanone (formula II)
[0049]
[0050] 1. Preparation of 2-bromo-1-(5-fluoro-2-hydroxyphenyl)ethanone
[0051]
[0052] Add 5-fluoro-2-hydroxyacetophenone (160g, 1.03mol) and copper bromide (500g, 2.24mol) into a 3L three-neck flask, then add ethyl acetate (700mL) and dichloromethane (700mL), and stir The temperature was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com