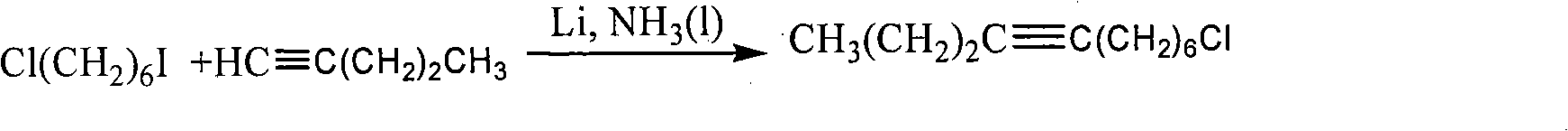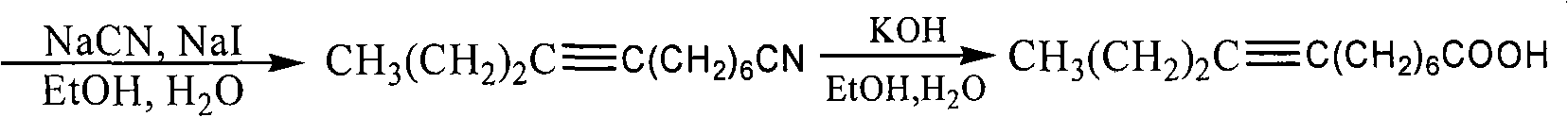Method of preparing cydia molesta sex pheromone
A technology of sex pheromone and alcohol acetate of pear borer moth, which is applied in the field of preparation of sex pheromone of pear borer moth, and can solve problems such as difficulty in obtaining terminal alkyne, long route, harsh reaction conditions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
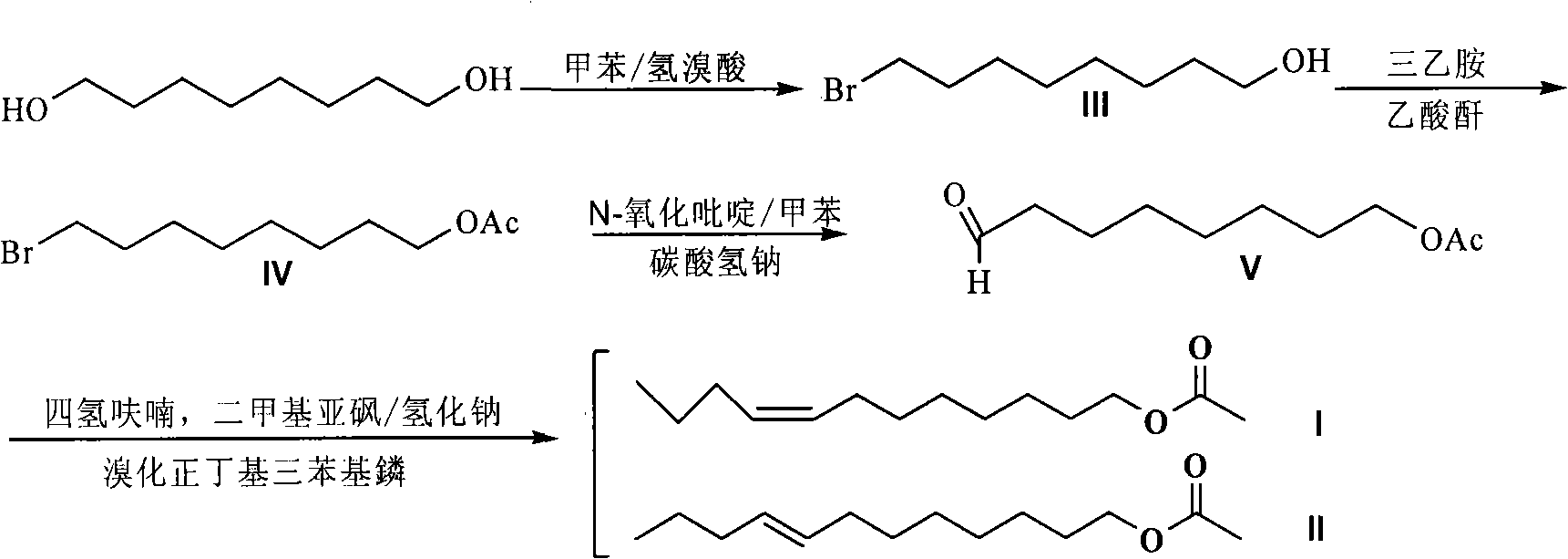
[0034] The synthesis of a, 8-bromo-1-octanol (III)
[0035] Add 14.6g (0.1mol) of 1,8-octanediol and 250mL of toluene into a 500mL round-bottomed flask, add 12.6mL of hydrobromic acid with a concentration of 40% dropwise for 10 minutes, and then reflux the system at 115°C for 10 minutes hour, then the system was lowered to room temperature, and 10 mL of hydrobromic acid with a concentration of 40% was added. After the addition was completed in 8 minutes, the system was divided into water and refluxed for 8 hours. TLC monitored the reaction process, and there was no raw material diol, only a small amount of dibromo , terminate the reaction, distill off the toluene, dissolve the residue with ether, and then use saturated NaHCO 3 The solution was washed with saturated brine, the organic layer was dried over anhydrous sodium sulfate, the solvent was removed, and silica gel column chromatography was used to obtain 18.03 g of 8-bromo-1-octanol (III) compound, a colorless liquid, wit...
Embodiment 2
[0043]The synthesis of a, 8-bromo-1-octanol (III)
[0044] Add 7.3g (0.05mol) of 1,8-octanediol and 150mL of toluene into a 250mL round-bottomed flask, add 13mL of hydrobromic acid with a concentration of 40% dropwise for 10 minutes, and then reflux the system at 115°C for 15 hours , then the system was lowered to room temperature, and TLC monitored the reaction process. There was no raw material diol, only a small amount of dibromo, the reaction was terminated, the toluene was evaporated, and the residue was dissolved in ether, followed by saturated NaHCO 3 The solution was washed with saturated brine, the organic layer was dried over anhydrous sodium sulfate, solvent removed, and separated by silica gel column chromatography to obtain 8.66 g of 8-bromo-1-octanol (III) compound, a colorless liquid, with a yield of 83.2%;
[0045] B, the synthesis of 1-acetoxy-8-bromooctane (IV)
[0046] Weigh 4.16g (20mmol) of 8-bromo-1-octanol (III) in a 250mL round bottom bottle, add solve...
Embodiment 3
[0052] The synthesis of a, 8-bromo-1-octanol (III)
[0053] Add 11.68g (0.08mol) of 1,8-octanediol and 150mL of toluene into a 250mL round-bottomed flask, add 11mL of hydrobromic acid with a concentration of 40% dropwise for 10 minutes, and then reflux the system at 115°C for 10 hours , then the system was lowered to room temperature, and 8 mL of 40% hydrobromic acid was added. After the addition of 8 minutes, the system was divided into water and refluxed for 8 hours. TLC monitored the reaction process. There was no raw material diol, only a small amount of dibromo, The reaction was terminated, the toluene was evaporated, and the residue was dissolved in ether, followed by saturated NaHCO 3 The solution was washed with saturated brine, the organic layer was dried over anhydrous sodium sulfate, solvent removed, and separated by silica gel column chromatography to obtain 14.00 g of 8-bromo-1-octanol (III) compound, a colorless liquid, with a yield of 86.1%;
[0054] B, the syn...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com