Method for preparing vitamin A acetate through one-pot method
A technology of acetate and vitamin, which is applied in the field of preparing vitamin A acetate in one pot, can solve the problems of excessive waste water and waste, unfavorable industrial production, complicated reaction steps, etc., and achieve the goal of improving industrial production level and reducing solvents Use and separation, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
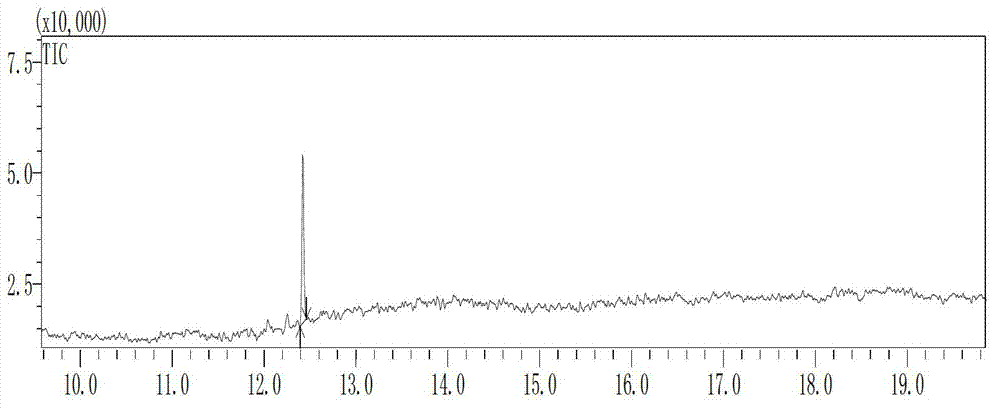
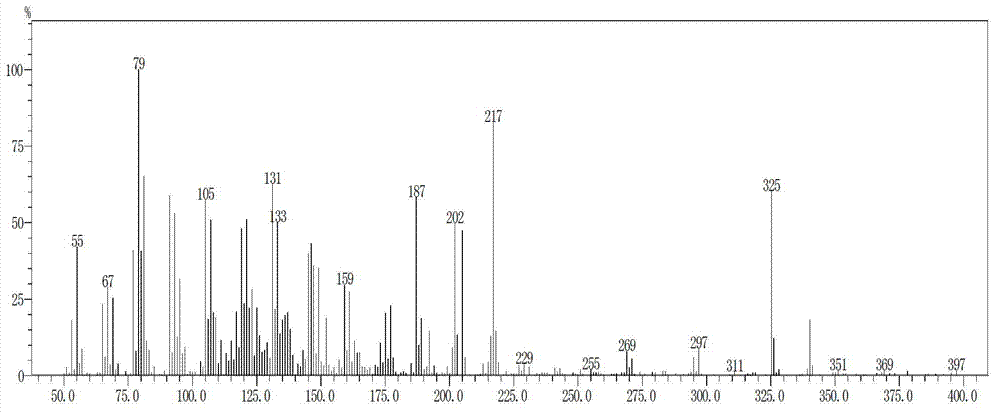
[0063] Under the protection of nitrogen, add 200 grams of N,N-dimethylformamide and 20.4 grams (0.3 moles) of solid sodium ethylate to a dry 1000 ml glass flask in sequence, cool and keep the temperature between -5 °C and 5 °C. Add dropwise a mixed solution prepared by 20.6 g (0.1 mole) of C14 aldehyde, 34.6 g (0.12 mole) of tetraethyl methylene diphosphonate and 100 g of N,N-dimethylformamide for about 2 hours Finished adding. Thereafter, the reaction was carried out at -5°C~5°C for 2.5 hours, and the conversion rate of C14 aldehyde was 99.9% by gas phase detection. The resulting intermediate C15 phosphonate was confirmed by GC-MS analysis, see figure 1 and figure 2 .
[0064] After the conversion of C14 aldehyde is completed, the reaction product intermediate does not need to be separated, and the reaction solution is cooled to -20°C, and then 17.0 grams (0.12 moles) of C5 aldehyde and 50 grams of N, N-dimethyl For the mixed solution of formamide, the dropwise addition ...
Embodiment 2
[0066] Under the protection of nitrogen, add 300 grams of N,N-dimethylformamide and 33.6 grams (0.3 moles) of potassium tert-butoxide solid to a dry 1000 ml glass flask in sequence, cool and keep the temperature between -5°C and 5°C , add dropwise a mixed solution prepared by 20.6 g (0.1 mole) of C14 aldehyde, 34.6 g (0.12 mole) of tetraethyl methylene diphosphonate and 100 g of N,N-dimethylformamide for about 2 hours The dropwise addition is completed; the insulation reaction takes 2 to 3 hours. After gas-phase detection of C14 aldehyde conversion is complete, the reaction solution is continued to cool down to -20°C, and then a mixed solution of 17.0 grams (0.12 moles) of C5 aldehyde and 50 grams of N,N-dimethylformamide is added dropwise, and the dropping temperature is controlled at -10°C~-15°C, the dropwise addition is completed in about 1 hour. After the dropwise addition, it is kept at -5°C for 2 to 3 hours. ℃), recover the solvent until no material evaporates, cool to ...
Embodiment 3
[0068] Under the protection of nitrogen, add 200 grams of N,N-dimethylformamide and 21.6 grams (0.4 moles) of sodium methoxide solid to a dry 1000 ml glass flask successively, cool, keep the temperature between -5°C and 5°C, drop Add a mixed solution prepared by 20.6 g (0.1 mole) of C14 aldehyde, 34.6 g (0.12 mole) of tetraethyl methylene diphosphonate and 100 g of N, N-dimethylformamide, dropwise for about 2 hours complete. Thereafter, the reaction was incubated for 2 to 3 hours. After gas-phase detection of C14 aldehyde conversion is complete, the reaction solution is continued to cool down to -20°C, and then a mixed solution of 17.0 grams (0.12 moles) of C5 aldehyde and 50 grams of N,N-dimethylformamide is added dropwise, and the dropping temperature is controlled at -10°C~-15°C, the dropwise addition is completed in about 1 hour. After the dropwise addition, it is kept at -5°C for 2 to 3 hours. ℃) Recover the solvent until no material evaporates, cool to room temperature...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com