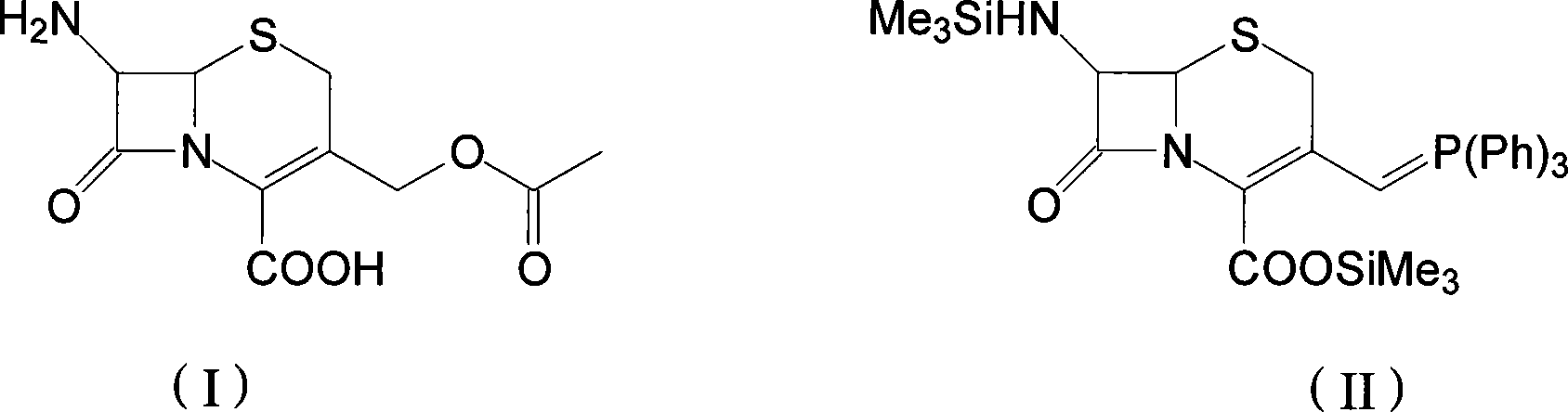Method for preparing cephalosporin propylene
A technology of cefprozil and propylene, which is applied in the field of preparation of antibiotic drugs, can solve problems such as unstable quality, low yield, and difficulty in obtaining raw materials, and achieve the effects of high yield, low cost, and reduced loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] 7-Trimethylsilylamino-3-triphenylphosphonomethylene-4-cephalosporanic acid trimethylsilyl ester
[0036] Add 100 mL of diethyl ether into a clean 250 mL reaction flask, add 12 g of 7-ACA, 12.6 g of BSA, and 0.15 g of imidazole with stirring, and heat to 37°C under the protection of an inert gas, and maintain reflux for 7 hours. After the reflux was completed, the material was cooled to 5°C, and 13.5 g of triphenylphosphine was added, followed by 8.8 g of iodotrimethylsilane, and the temperature was maintained at 4°C. React at this temperature for about 6 hours at a constant temperature. The disappearance of the raw material 7-ACA as detected by liquid chromatography is the end of the reaction, and the compound 7-trimethylsilylamino-3-triphenylphosphonomethylene-4-cephalosporanic acid is obtained A mixed solution of trimethylsilyl ester (II).
Embodiment 2
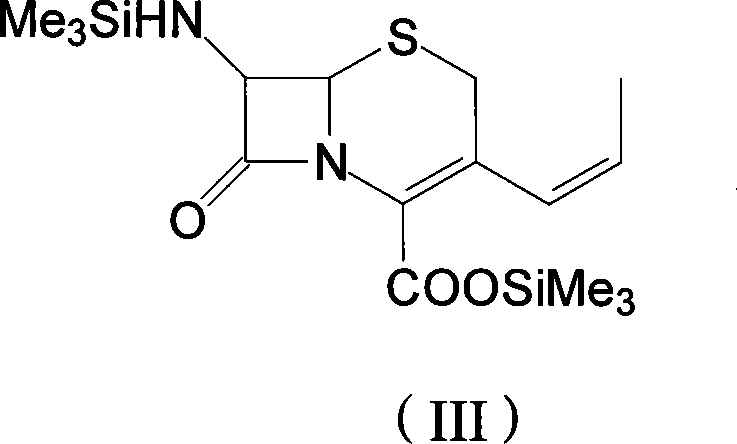
[0037] Example 2 7-trimethylsilylamino-3-(prop-1-enyl)-4-cephalosporanic acid trimethylsilyl ester
[0038] Keep the temperature of the mixed solution in step 1 between 4±1°C, add 5g of dry phenyllithium to it, stir for 20 minutes, then add 10.5mL of anhydrous acetaldehyde, control the temperature at -3±1°C for 15 hours , until the disappearance of compound (II) is the end point of the reaction to obtain a mixed solution of compound 7-trimethylsilyl amino-3-(prop-1-enyl)-4-cephalosporanic acid trimethylsilyl ester (III) , cooled to -15°C for later use.
Embodiment 3
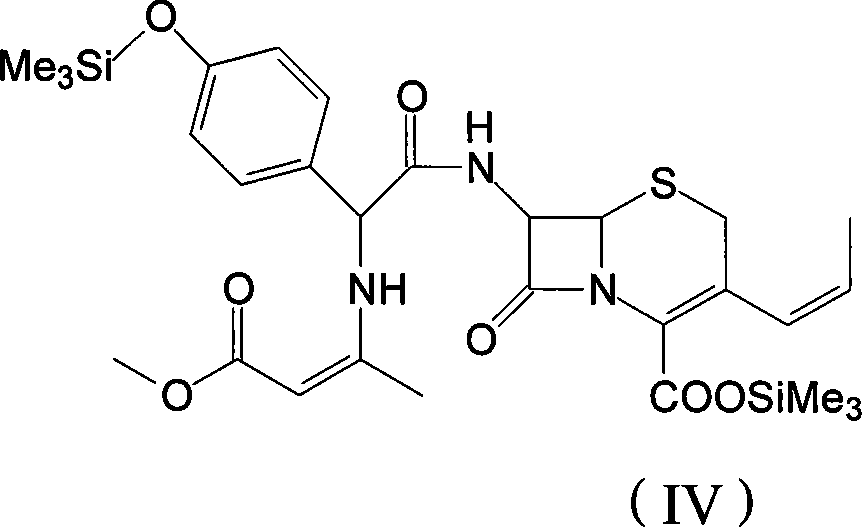
[0040] Add 50mL of dichloromethane into a clean 100mL reaction bottle, then add 5.2g of D-p-hydroxyphenylosysine Deng potassium salt at one time, stir for 10 minutes, cool down to -50°C, add DMF 10mL, methanesulfonic acid 0.05g, N-methylmorpholine 0.01g, continue to cool to -60°C, add 1.8g of ethyl chloroformate, keep the reaction at -40°C for 2 hours, then immediately add the mixed solution in the 100mL reaction bottle to the step In the mixed solution of 2, the temperature was slowly raised to -5°C, reacted for 30 minutes, and then slowly raised to room temperature to obtain a mixed solution of compound (IV).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com