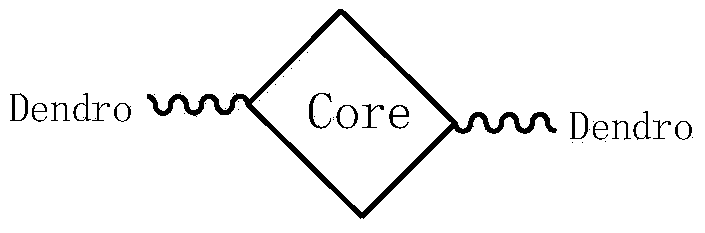
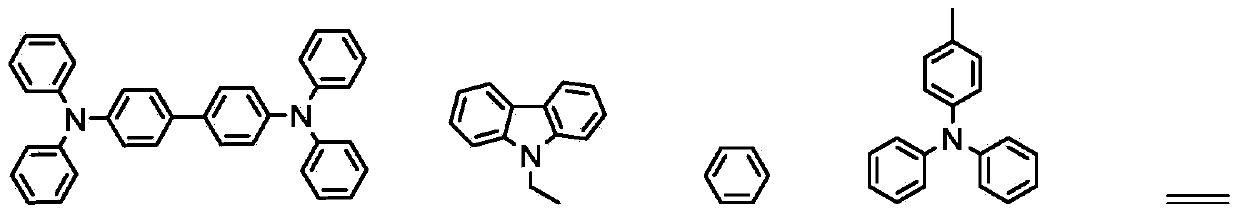
Soluble triphenylamine type organic micromolecular hole transport material and preparation method and application thereof
A technology of hole transport material and triphenylamine, which is applied in the field of organic electroluminescent display, can solve the problems of low efficiency, poor solubility, and shortened lifespan, and achieve the effects of simple synthesis route, convenient purification, and good film-forming properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: Synthesis of 4-(N,N-di-p-methylphenyl)aminobenzyl alcohol (compound 1)
[0033] Add 15.12g (50mmol) 4-(N,N-bis(4-methylphenyl)amino)benzaldehyde, 150mL dichloromethane (CH 2 Cl 2 ) and 50mL absolute ethanol (Et 2 OH) mixed solvent, after stirring and dissolving, add 1.91g (0.02mol) sodium borohydride (NaBH 4 ), stirred and reacted at room temperature (25°C) for 2 hours, and monitored the completion of the reaction with TLC (silica gel GF254 plate, developer: petroleum ether: ethyl acetate = 5:1), evaporated the solvent under reduced pressure, and distilled the product with 50mL CH 2 Cl 2 After dissolving, wash three times with deionized water, combine the organic layers and add anhydrous MgSO 4 After drying, the solvent was evaporated under reduced pressure to obtain a green viscous liquid. Compound 1 (white solid, 13.62 g, yield 90%) was obtained after solidification with n-pentane.
Embodiment 2
[0034] Embodiment 2: the synthesis of triphenylphosphine hydrobromide (compound 2)
[0035] Add 100mL tetrahydronaphthalene to a 250mL four-neck flask, slowly drop in 40mL bromine (Br 2 ), catalyzed by a small amount of Fe powder, bubbles are generated, after washing the bottle of HBr gas generated by tetrahydronaphthalene, it is passed into 500mL ether solution containing 21.03g (0.08mol) triphenylphosphine, and stirred at room temperature to generate The white triphenylphosphine hydrobromide solid was suction filtered after the reaction, the product was washed with diethyl ether, and then recrystallized with dichloromethane and diethyl ether to obtain the product (white solid, 17.48g, yield 64%). Mp.200~202℃.
Embodiment 3
[0036] Example 3: Synthesis of 4-(N,N-di-p-methylphenyl)aminobenzyltriphenylphosphine hydrobromide (compound 3)
[0037] N 2 Under protection, add 2.39g (8mmol) 4-(N,N-di-p-methylphenylamino) benzyl alcohol and 5.40g (16mmol) triphenylphosphine hydrobromide to a 100mL four-neck flask, add 60mL Chloroform (CHCl 3 ) as a solvent, stirred and dissolved, heated to reflux temperature (61°C) and stirred for 4 hours. TLC (silica gel GF254 plate, developer is petroleum ether: ethyl acetate = 5:1) to monitor the completion of the reaction, the solvent was evaporated under reduced pressure to obtain a light green oil, which was washed with ether and solidified to obtain a white powder product compound 3 ( White solid, 4.77g, yield 96%).
[0038] Synthesis of 4-(N,N-Di-p-methylphenyl)aminophenylallyltriphenylphosphine Hydrobromide
[0039]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Maximum current efficiency | aaaaa | aaaaa |
| Maximum brightness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com